Monitoring Of Na Treatment
Detection of the following relevant indicators at baseline before treatment
major biochemical markers, such as ALT, AST, bilirubin, and albumin major virological and serological markers, such as HBV DNA quantification, HBsAg, HBeAg, and anti-HBe blood routine serum creatinine levels, blood phosphorus levels, and renal tubular function should be tested if required noninvasive tests for liver fibrosis, such as liver stiffness measurement and when ETV and TDF are used in patients with creatinine clearance < 50 mL/min, the doses of both drugs should be adjusted. There is no recommended dose for TAF when it is used in patients with creatinine clearance < 15 mL/min who are not receiving hemodialysis. In other cases, no dose adjustment is required.
Patient compliance
It should be closely monitored, which includes dosage, method of use, missed medication or self-discontinuation, to ensure that patients understand the risks that might result from unwarranted discontinuation and improve their compliance.
Prevention and management of rare adverse events
Monitoring and management of drug resistance
The use of potent antiviral drugs with minimal resistance has resulted in significantly reduced rates of resistance that could arise from long-term treatment of NAs. If HBV DNA levels increased > 2 lg IU/mL from nadir during treatment and the potential of poor compliance has been ruled out, salvage therapy should be initiated promptly, and drug resistance should be tested for.
Efficacy And Safety Of Nas
Entecavir
Numerous studies have shown that the use of ETV is safe and highly effective to suppress virus replication and reduce liver inflammation.117119 Long-term treatment with ETV can improve histological changes in patients with cirrhosis,120,121 significantly reduce the incidence of cirrhosis-related complications and HCC, and reduce liver-related and all-cause mortality.53,122 The 5-year cumulative probability of ETV resistance was 1.2% in treatment-naïve patients with CHB but increased to 51% in CHB patients who were resistant to lamivudine .123
Tenofovir disoproxil fumarate
Multicenter clinical studies of TDF treatment for CHB patients have shown that it can strongly inhibit virus replication and has low rates of resistance.124,125 A study of TDF usage for 8 years showed that there were 41 cases of virological breakthroughs, 29 cases of which were due to poor compliance. In total, 59% of patients with virological breakthroughs continued to receive TDF treatment and achieved virological responses. Further nucleic acid sequencing did not identify TDF-related resistance.126 Long-term treatment with TDF can significantly improve liver histology and reduce the incidence of HCC.127,128
Tenofovir alafenamide fumarate tablets
Other drugs
Telbivudine can improve eGFR but it is associated with high rates of resistance.113 LdT exhibits favorable efficacy and safety in blocking mother-to-child transmission .
Qhbsag As A New Marker Of Treatment Efficacy
Serum concentrations of qHBsAg, which reflect levels of cccDNA in the liver, vary during the course of CHB they are highest in the immune-tolerant phase, followed by a decline during the immune clearance phase and a further decrease after HBeAg seroconversion, becoming lowest in inactive carriers. With IFN-based antiviral therapy, a rapid reduction in qHBsAg is predictive of a sustained response thus, an early stopping rule has been proposed, suggesting that IFN therapy can be stopped or switched by week 12 in patients without qHBsAg decline because they are unlikely to achieve a response with further IFN treatment. In NUC-based therapy, the clinical relevance of qHBsAg is less well defined. qHBsAg reductions are generally less pronounced with NUCs compared with IFNs, and the data regarding a potential association of qHBsAg with serologic or virologic responses are inconsistent. Thus, more research is needed to understand qHBsAg kinetics during NUC therapy and allow potential tailoring of treatment duration to individual patients.
Don’t Miss: How Is Hepatitis C Virus Transmitted
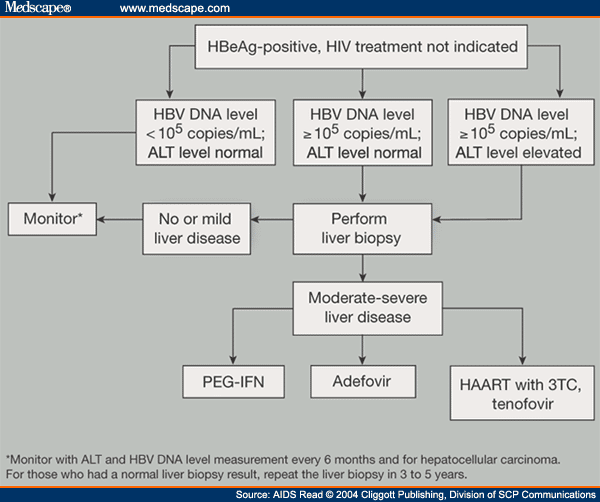
Indications For Antiviral Therapy
Patients should be assessed for the risk of disease progression to determine whether to start antiviral therapy based on a comprehensive analysis of serum HBV DNA levels, ALT levels, the severity of liver disease, as well as their age, family history, and concomitant diseases.6,112,113 Dynamic assessment is more meaningful than a single test .
Flow chart of antiviral therapy for patients with chronic HBV infection.
Follow-up tests: virological test, liver biochemical test, AFP test, PIVKA-II test, abdominal ultrasound, and liver stiffness measurement. HBV-related extrahepatic manifestations: glomerulonephritis and vasculitis. Follow-up criteria for patients with HBV-related decompensated cirrhosis during NA treatment: perform a routine blood test, liver biochemical test, renal function test, virological test, and test for blood ammonia, AFP, and PIVKA-II, and abdominal ultrasound every 3 months. If necessary, perform enhanced CT or MRI. Other causes of ALT elevation: infection by other pathogens, history of drug or poison use, history of alcohol use, lipid metabolism disorders, autoimmune disorders, liver congestion or vascular diseases, inherited metabolic liver disease, systemic diseases. NAs-ETV, TDF, TAF. HbsAg, hepatitis B surface antigen HBV, hepatitis B virus ALT, alanine aminotransferase HCC, hepatocellular carcinoma NAs, nucleoside analogs peg-IFN-, pegylated interferon-.
Cases And Clusters Of Potential Public Health Importance
Jurisdictions should review and analyze hepatitis B data regularly to identify cases and clusters of hepatitis B that merit further investigation. When resources are limited, these should be prioritized for investigation based on the degree of public health importance. The following are examples of high priority cases and clusters:
- People of childbearing age who are or have the potential to become pregnant, indicating the potential for perinatal transmission
- Children 24 months of age to detect perinatal transmission
- People in age and demographic groups for whom infection may be acute due to recent transmission, including those
- 70 years of age
Also Check: Can Hepatitis C Cause Diarrhea
Management Of Chronic Hbv Carriers And Inactive Hbsag Carriers
Because chronic HBV carriers are in the immune tolerance phase, they do not have or only have mild inflammatory activity in the liver, patients in this phase respond poorly to antiviral treatment. Therefore, antiviral therapy is not recommended. However, it is emphasized that some patients in the immune tolerance phase might enter the immune clearance phase and develop hepatitis flares. Inactive HBsAg carriers are in the immune control phase, but they might progress to HBeAg-negative CHB and are at risk of developing HCC in long-term follow-up.
Therefore, for chronic HBV carriers and inactive HBsAg carriers, it is recommended that they should undergo routine blood tests, biochemical tests, virological tests, AFP tests, abdominal ultrasound, and noninvasive tests for liver fibrosis every 612 months. A liver biopsy is recommended if necessary. Antiviral therapy should start if they meet the indications for such treatment.
What Causes Hepatitis B
- being born to a mother with hepatitis B
- having unprotected sex with an infected person
- sharing drug needles or other drug materials with an infected person
- getting an accidental stick with a needle that was used on an infected person
- being tattooed or pierced with tools that were used on an infected person and werent properly sterilized, or cleaned in a way that destroys all viruses and other microbes
- having contact with the blood or open sores of an infected person
- using an infected persons razor, toothbrush, or nail clippers
You cant get hepatitis B from
- being coughed on or sneezed on by an infected person
- drinking unclean water or untreated water that has not been boiled
- eating food that is unclean or has not been properly cooked
- hugging an infected person
- shaking hands or holding hands with an infected person
- sharing spoons, forks, and other eating utensils
- sitting next to an infected person
Mothers who have hepatitis B can safely breastfeed their babies. If a baby receives hepatitis B immune globulin and starts receiving the hepatitis B vaccine to prevent hepatitis B infection shortly after birth, hepatitis B is unlikely to spread from mother to child through breastfeeding.15
You May Like: Hepatitis C And Kidney Failure
Read Also: Is Chronic Hepatitis C Curable
Virological Testing For Hbv
HBV DNA quantification
This is mainly used to assess the level of virus replication in HBV-infected patients. In addition, it can be used as a crucial component to select the indications and assess the efficacy of antiviral therapy. During antiviral therapy, obtaining a sustained virological response could significantly control the progression of cirrhosis and lower the risk of HCC.53,54 The quantitative detection of HBV DNA utilizes real-time quantitative polymerase chain reaction however, the detection limit varies between manufacturers reagents.
HBV genotyping
To date, at least nine HBV genotypes and one undefined genotype have been identified. Some genotypes are further divided into subtypes. The detection of HBV genotypes could help to predict the efficacy of IFN and determine the disease prognosis.5558
Detection of resistant mutants
Treatment Duration And Stopping Rules
For NUC-based antiviral therapy, the guidelines stipulate that treatment can be stopped after achieving certain end points that reflect the patient’s HBeAg status and degree of liver fibrosis. In HBeAg-positive patients, the recommended end points are HBeAg seroconversion following sustained undetectable HBV DNA with ALT normalization in HBeAg-negative patients or HBeAg-positive patients who do not seroconvert, the recommended end points are sustained undetectable HBV DNA with ALT normalization. Consolidation therapy is recommended in patients who achieve these end points, but there is no consensus on its optimal duration . Ultimately, HBsAg loss is the ideal end point because it is associated with a significantly reduced HCC risk, although not as low as that of a person who has never been infected with HBV.
IFN-based therapy is administered over a finite duration , irrespective of achievement of these end points, since prolonged maintenance therapy to suppress HBV replication is not feasible with these regimens.
Also Check: Hepatitis B Surface Antibody Low
Special Considerations During Immunosuppressive Therapy
With immunosuppressive therapy, both in the context of malignancy and rheumatologic/autoimmune diseases, reactivation of HBV infection can occur. HBV reactivation in HIV-negative people with HBsAg-positive/anti HBc-positive disease receiving immunomodulatory therapy is well described.147,148 Even among people with HBsAg-negative/anti-HBc-positive disease, HBV reactivation occurs in 8% to 18% of people receiving anti-cancer drugs149 and 1.7% of people receiving rheumatologic disease drugs.150
What Are The Treatment Options For Chronic Hep B
For people with acute hep B infection experiencing mild symptoms, doctors often recommend rest, a healthy diet, and fluids to speed up recovery. Severe symptoms may need to be treated in a hospital.
According to the Hepatitis B Foundation, there are currently seven drugs approved by the FDA to treat chronic hep B in the United States. Not everybody needs to take medication, but some people will need to take medication for the rest of their lives.
These drugs fall into one of two categories:
- Antiviral drugs. These drugs help reduce inflammation and liver damage. Theyre usually taken daily in pill form for at least a year.
- Immune modulator drugs. These drugs boost your immune system to help your body fight off the virus. Theyre administered as an injection over 6 to 12 months.
Theres no cure for hep B, acute or chronic, at the moment. However, clinical trials continue to investigate new treatment options.
You May Like: How Is Hepatitis A Caused
Occult Hbv Infection 107
Patients are negative for HBsAg in serum but positive for HBV DNA in serum or liver tissue, or both. A total of 80% of OBI patients might be seropositive for anti-HBs, and anti-HBe or anti-HBc, or both, which is designated as seropositive OBI however, 120% of OBI patients are seronegative for all serological indicators, which is designated as seronegative OBI. Its mechanism has not been determined. One possible explanation is that HBsAg disappears after apparent HBV infection, and HBV DNA levels are usually very low in the serum or liver tissue, without obvious liver tissue damage. Another possibility is mutations in the S gene region of HBV, which makes HBsAg undetectable by currently available commercial kits. Serum HBV DNA levels are usually high, which might be accompanied by significant liver histopathological changes. These patients can transmit HBV to recipients through blood transfusion or organ transplantation and might experience reactivation of HBV if they are immunosuppressed.
Chronic Hepatitis B Complications

Chronic hepatitis B can lead to
- cirrhosis, a condition in which scar tissue replaces healthy liver tissue and prevents your liver from working normally. Scar tissue also partly blocks the flow of blood through the liver. As cirrhosis gets worse, the liver begins to fail.
- liver failure, in which your liver is badly damaged and stops working. Liver failure is also called end-stage liver disease. People with liver failure may require a liver transplant.
- liver cancer. Your doctor may suggest blood tests and an ultrasound or another type of imaging test to check for liver cancer. Finding cancer at an early stage improves the chance of curing the cancer.
Read Also: Why Does Hepatitis Cause Joint Pain
Exposure To A Source With Unknown Hbsag Status
Unvaccinated persons and persons with previous nonresponse to hepatitis B vaccination who have a discrete, identifiable exposure to blood or body fluids containing blood from a person with unknown HBsAg status should receive the hepatitis B vaccine series, with the first dose initiated as soon as possible after exposure and the series completed according to the age-appropriate dose and schedule. Exposed persons who are not fully vaccinated but started the series should complete the vaccine series. Exposed persons with written documentation of a complete hepatitis B vaccine series who did not receive postvaccination testing require no further treatment.
Serological Testing For Hbv
Traditional serum markers for HBV include HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, and anti-HBc immunoglobulin M. Serum HBsAg can be produced by cccDNA-derived mRNA or an HBV DNA sequence that is integrated into the human genome. HBsAg positivity indicates HBV infection. Anti-HBs is a protective antibody, and anti-HBs positivity indicates immunity to HBV, which can be seen in people with a resolved hepatitis B infection or who have been inoculated with the hepatitis B vaccine. Anti-HBc-IgM positivity is mostly found in patients with acute hepatitis B. Anti-HBc-IgM is usually mildly positive in patients with reactivation of chronic HBV infection. The main subtype of anti-HBc is IgG. The IgG subtype is positive in most HBV cases, whether the virus is eliminated or not.
Recently, the quantitative detection of HBsAg has been widely used in clinical practice. Its level is indicative of the stage of the disease and the risk of disease progression. In addition, it can be used to guide the use of recombinant human IFN and peginterferon- .
You May Like: Hepatitis B Home Test Kit
Controversial Issues In Clinical Practice
Nucleoide analogs withdrawal and retreatment
Oral nucleoide analogs used in first-line treatment, such as TDF, entecavir , and tenofovir alafenamide fumarate , have strong anti-virus effects, fewer side effects, convenience, and low resistance. However, long-term duration, especially > 10 years of administration, requires intensive focus on drug safety and may result in reduced compliance in patients . Drug withdrawal can be considered in HBeAg+ patients after e-antigen seroconversion and consolidation therapy, as recommended by guidelines . Conversely, for HBeAg- patients, the 2019 China guidelines recommended drug withdrawal upon serum HBsAg disappearance and HBV DNA below the limit of detection, and the 2018 AASLD guidelines recommended at least 2 years of viral inhibition and consolidation therapy, while the 2017 EASL guidelines recommended a minimal 3-year viral inhibition. The 2015 APASL guidelines recommended drug withdrawal upon serum HBsAg disappearance, followed by 12-month consolidation therapy or undetectable HBV DNA and a minimum of 2 years of treatment.
Low level viremia
Table 3. Recommendations for salvage therapy in resistant patients in 2019 guideline.
Inactive Hbsag Carrier State105106
This is also known as HBeAg-negative chronic HBV infection. Patients are in the immune control phase. They are positive for HBsAg and anti-HBe and negative for HBeAg. HBV DNA levels are < 2,000 IU/mL, and HBsAg levels are < 1,000 IU/mL. ALT and AST levels are persistently normal . Imaging examination shows no signs of cirrhosis. Liver biopsy shows a histological activity index score < 4, or lesions are mild using other semi-quantitative scoring systems.
Also Check: Which Hepatitis Causes Liver Cancer
Hepatitis B And Coinfections
Patients with hepatitis B can be monoinfected or coinfected. The most common hepatitis B coinfections are:
Coinfection with HDV
A) HBV/HCV coinfection
Because they share similar transmission routes, HBV/HCV coinfection is more common in regions endemic for both viruses. The global prevalence of this coinfection ranges from 1% to 15%. Some studies have shown that HCV is usually dominant and HBV can be serologically evident or occult.
Recommendations
1 Before starting therapy with DAAs, patients infectedwith HCV should be tested for HBV coinfection usingHBsAg and for past infection using anti-HBs and anti-HBc .
2 HBsAg-positive patients who do not meet the criteriafor HBV treatment should receive antiviral prophylaxisfor HBV for at least 12 weeks after hepatitis C treat-ment .
3 In HBsAg-negative and anti-HBc-positive patients,serum ALT levels should be monitored monthly. If ALTis elevated, the patient should be retested for HBsAgand HBV DNA .
4 If HBsAg and/or HBV DNA become positive after theuse of DAAs, HBV treatment should be initiated .
5 The antiviral drugs of choice for the treatment of HBVin HBV/HCV coinfection are entecavir, tenofovir diso-proxil, and tenofovir alafenamide .
B) HBV/HIV coinfection
Recommendations
1 All HBV/HIV-coinfected patients should receive antivi-ral therapy that includes two medications activeagainst HBV, specifically tenofovir combined withlamivudine or emtricitabine .
References
C) HBV/HDV coinfection
References
Recommendations