Hepatitis Serologic Analysis And Hbv Dna Assay
HBV serologic markers, including HBsAg, HBsAb, HBeAg, HBeAb, anti-HCV Ab , antiâhepatitis-D antibody, and anti-HIV antibody, were tested by using commercially available enzyme immunoassays . HBcAb was tested by radioimmunoassay . Serial serum samples from the same patient were tested in a single run to minimize interassay variation. A nested PCR assay for detection of serum HBV DNA was done by using primer sets from the HBV surface antigen and the core antigen coding region. For the surface region, the primers were CCTGCTGGTGGCTCCAGTTC and CAAACGGGCAACATACCTTG for the first round of PCR testing and ACATCAGGATTCCTAGGACC and CGCAGACACATCCAGCGATA for the second round. The corresponding sets of primers for the core region were GGAGTGTGGATTCGCATCCTCC , ATACTAACATTGAGATTCCC , AGACCACCAAATGCCCCTAT , and GATCTTCTGCGACGCGGCGA . Using serial dilution of EuroHep-2 HBV DNA plasma standards, we estimated the detection limit of the PCR assay to be 1âÃâ102 genomes/mL. Results of the Digene Hybrid Capture II assay were expressed in copies per milliliter, and the manufacturer’s stated cut-off limit for detecting HBV viremia in clinical specimens was 0.142âÃâ106 copies/mL. Reverse transcriptionânested PCR for HCV was done in a single tube assay as described previously. The outer primers 57 and 321 and the inner primers 126 and 299 were designed from the highly conserved 5â² noncoding region.
High Hepatitis B Virus Dna Viral Load As The Most Important Risk Factor For Hbv Reactivation In Patients Positive For Hbv Surface Antigen Undergoing Autologous Hematopoietic Cell Transplantation
George K. K. Lau, Yu-hung Leung, Daniel Y. T. Fong, Wing-yan Au, Yok-lam Kwong, Albert Lie, Ji-lin Hou, Yu-mei Wen, Amin Nanj, Raymond Liang High hepatitis B virus DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002 99 : 2324â2330. doi:
Adverse Effects Of Treatment
Adherence to treatment remains a major factor influencing the rates of sustained virologic response.33,42 Discontinuation of therapy because of adverse events is common and has been reported in up to one third of patients. Approximately 50 to 60 percent of patients may exhibit self-limited influenza-like symptoms with interferon-based therapy.2 Effective management of treatment-related adverse events is essential to improve adherence to treatment therefore, patients should be monitored closely for hematologic, renal, and thyroid abnormalities. Approximately 30 percent of patients undergoing treatment for HCV infection experience depression, emotional lability, or anger, but treatment is rarely associated with suicidal ideation or hallucinations.43 Treatment for HCV infection is contraindicated in persons with uncontrolled major depression.32 A recent randomized trial found that the overall adverse effects of pegylated interferon alfa-2b plus ribavirin and pegylated interferon alfa-2a plus ribavirin were similar.40 Adverse effects of pegylated interferon and ribavirin for the treatment of HCV infection are listed in Table 8.32
Adverse Effects of Pegylated Interferon and Ribavirin for the Treatment of Hepatitis C Virus Infection
Information from reference 32.
Adverse Effects of Pegylated Interferon and Ribavirin for the Treatment of Hepatitis C Virus Infection
Information from reference 32.
Recommended Reading: What Hepatitis Vaccines Are Available
Premature Rupture Of The Membranes
Premature rupture of the membranes is considered a risk factor for HCV vertical transmission by exposing the fetus to maternal HCV in the birth canal. The duration of rupture has been found to be significantly longer in infected children. These parameters are potentially related to contamination of the fetus with infected maternal blood in the birth canal.
Enzyme Immunoassays For Detection Of Hepatitis C Antibody
The HCV Ab test is used for initial screening for hepatitis C. The test is performed by enzyme immunoassays , which detect the presence of hepatitis C antibodies in serum. The result of the test is reported as positive or negative. Third-generation EIAs have a sensitivity/specificity of approximately 99%. However, the presence of HCV Ab does not indicate whether the infection is acute, chronic, or resolved. A positive antibody test result should be followed up with an HCV RNA test to confirm that viremia is present.
You May Like: How To Check For Hepatitis
Pretransplantation Characteristics Associated With Hbv
Pretransplantation characteristics associated with hepatitis due to HBV reactivation after HCT were an elevated serum ALT level, HBsAg positivity, detectable serum HBV DNA , and BCP . Three patients with elevated serum ALT before HCT and 10 patients with normal serum ALT before HCT had hepatitis due to HBV reactivation . Eleven patients who were positive for HBsAg before HCT had hepatitis due to HBV reactivation, where only 2 HBsAg-negative patients had hepatitis due to HBV reactivation . Nine patients with detectable serum HBV DNA and only 4 without detectable serum HBV DNA had hepatitis due to HBV reactivation . Pretransplantation serum HBV DNA levels were also significantly higher in patients with hepatitis due to HBV reactivation than in those without hepatitis due to HBV reactivation . In addition, 7 patients with BCP and 5 without BCP had hepatitis due to HBV reactivation .
The Quantitative Hcv Rna Test Is Checked Before A Patient Starts Treatment
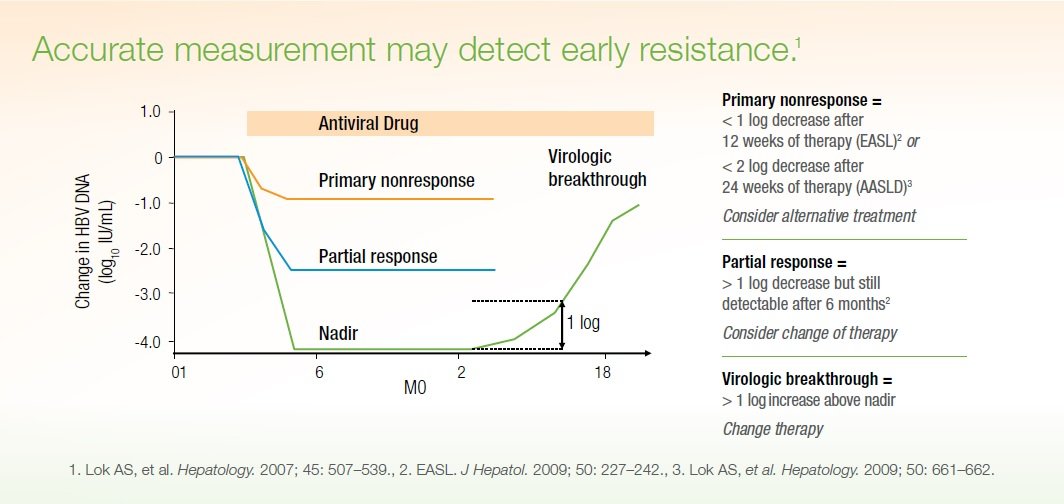
For each patient, the result can be described as either a “high” viral load, which is usually > 800,000 IU/L, or a “low” viral load, which is usually < 800,000 IU/L. It’s not uncommon to have a viral load in the millions. Today’s hepatitis C treatments are very effective with both high and low viral loads. An undetectable HCV viral load 10-12 weeks after hepatitis C is completed is associated with a cure.
Recommended Reading: Can Hepatitis Cause Low Platelets
Why Viral Load Is Important
Healthcare providers use your viral load to determine how well you’re responding to anti-viral treatment. Typically your viral load will be tested before you start therapy and then repeated periodically to measure how you are responding. At least two viral load results are needed to assess treatment efficacy.
A significantly reduced viral load, like a 100-fold decrease in viral actively, generally means that treatment is working. Ideally, a person would achieve a so-called “undetectable” viral load, meaning that the current testing technologies are unable to find any evidence of the virus in blood samples.
It’s important to understand that while the test is valuable in predicting treatment outcomes, it does not tell you anything about the severity of your liver disease. Typically, liver biopsies and imaging tests are needed for that.
Hcv Viral Load And New Treatments For The Hepatitis C Virus
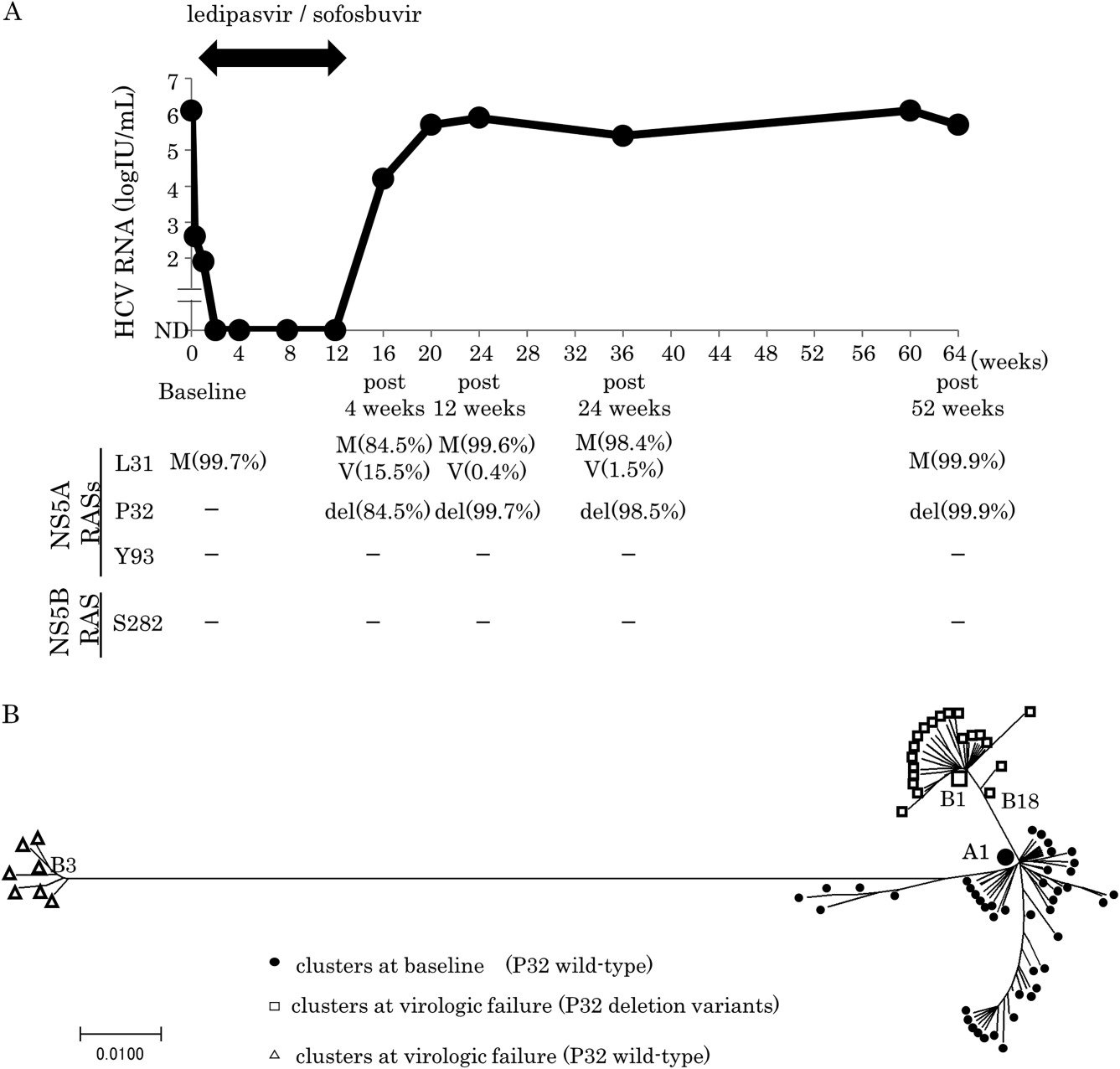
Hepatitis C treatment has significantly progressed in recent years thanks to better understanding of the virus replication cycle. Treatment is currently based on the use of direct acting antivirals targeting HCV viral proteins. A marketing authorisation has just been obtained for several DAAs targeting the hepatitis C virus and there has been major progress in the clinical development of several others. Thus, the standard treatment in 2015 is based on a combination of direct acting antivirals . Simeprevir and daclatasvir belong to the anti-protease group, sofosbuvir belongs to the anti-polymerases.
- Simeprevir: NS3/4A anti-protease
- Daclatasvir: NS5A anti-protease
- Sofosbuvir: NS5B anti-polymerase
These drugs should be prescribed for periods of 12 to 24 weeks according to whether or not they are in combination with ribavirin. The HCV genotype is also a factor in determining the form of treatment. Note that the current cost of treatment is very high and at present is reserved for patients with fibrosis and cirrhosis . The next step will to apply this treatment to patients infected with genotype 3 .
The assay of the viral genome is performed,
- just before commencing treatment,
- to monitor therapeutic efficacy by means of viral load kinetics using real-time PCR.
Recommended Reading: How To Reverse Hepatic Steatosis
Hepatitis C Virus Genotype And Its Correlation With Viral Load In Patients From Kathmandu Nepal
- Bhavesh Kumar MishraInstitute of Human Virology, Zhongshan School of Medicine, and Key Laboratory of Tropical Disease Control of Ministry of Education, Sun Yat-sen University, Guangzhou, China
- Uday Narayan YadavSchool of Medical Sciences, University of New South Wales, Sydney, Australia
- Saroj KhatiwadaForum for Health Research and Development, Dharan, Nepal
- Man Kumar TamangCentral Campus of Technology, Dharan, Nepal
- Shivir DahalCentral Campus of Technology, Dharan, Nepal
- Yi-Ping LiInstitute of Human Virology, Zhongshan School of Medicine, and Key Laboratory of Tropical Disease Control of Ministry of Education, Sun Yat-sen University, Guangzhou, China
Things To Know About Hepatitis C Viral Load
One way to keep track of a hepatitis C infection is by measuring your viral load. That’s the amount of the virus in your bloodstream. If you have hepatitis C, there are lots of things you need to know. For instance, what does your viral load mean for your treatment? And, why might you test positive for hepatitis C even if the virus is no longer in your system? Here are answers to those questions, and more.
You May Like: Hepatitis C Virus Antibody Reactive
Interpreting Hcv Rna Test Results
It is essential that the provider understands how to interpret HCV RNA test results, especially during the course of HCV treatment.
| Result of HCV RNA Test | Interpretation |
|---|---|
| A quantified viral load — any exact number | Ongoing HCV infection |
| “Detected” | The HCV RNA is detectable but the number of international units is so low that it cannot be quantified accurately. This indicates extremely low level of virus is present. |
| “< 12 IU/mL” or “< 15 IU/mL” or “< 25 IU/mL” All of these are “less than the LLOQ” | HCV RNA is undetectable. No virus is detected at all in the patient’s serum specimen. |
Hepatitis C In Children
Hepatitis C infection is a chronic viral infection of the liver that affects upwards of 1-2 percent of adults. Fortunately, in children and adolescents, hepatitis C is less common, but it remains a significant health issue. In this article I will address the most common questions about hepatitis C in children and adolescents.
What is the frequency of HCV in children and adolescents?
HCV occurs in about 0.15% of 6-11 year olds and 0.4% of 12-19 year olds. It is estimated that there are 23,000 to 46,000 children in the US with HCV. The recent opioid epidemic is leading to an increasing frequency in adolescents and young adults.
How do children acquire HCV?
Most children are infected with HCV at birth. This is called vertical transmission of infection . If a mother has HCV, her child has a 1 in 20 chance of becoming infected at birth. A high HCV viral load in the mother has a higher the risk of infection to her newborn infant. Interventions at birth, such as C-section delivery, have not been shown to alter the risk of infection at birth.
Adolescents acquire HCV in ways similar to adults by engaging in behaviors that increase their risk of blood exposure, such as IV drug use, sharing needles and high-risk sexual behaviors.
How do you diagnose HCV in children?
What happens to children who are infected with HCV?
What follow up is needed for a child with HCV?
Children with HCV should receive the hepatitis A and B vaccines. They should receive an annual influenza vaccine.
|
GENOTYPE |
Also Check: Medicine To Cure Hepatitis C
Clinical Characteristics Of Patients Undergoing Autologous Hct
Before HCT, 23 patients were positive for HBsAg, 37 were positive for anti-HBs , and 77 were negative for HBV. There were no significant differences in sex, age, underlying disease, conditioning regimen, or percentage of patients with elevated serum ALT levels before HCT .
Characteristics of 137 patients treated with autologous hematopoietic cell transplantation and evaluated for hepatitis due to hepatitis B virus reactivation, according to patients’ HBV status
| Characteristic . |
|---|
What The Qualitative Results Mean
The qualitative results indicate that HCV is present in your blood. The test result will be either detected or undetected.
Detected means that you do have the virus in your blood. Undetected means that you dont have the virus in your blood, or you have a tiny amount that cant be detected by this test.
The qualitative test results may still be positive even if your viral load has decreased drastically due to treatment.
Also Check: Does Hepatitis C Lower Your Immune System
Study Design And Population
A cross-sectional descriptive study took place from June 2016 to June 2019 at the Pietro Annigoni Centre for Biomolecular Research and the Laboratory of Molecular Biology and Genetics of the Joseph KI-ZERBO University in Ouagadougou, Burkina Faso. The study included 4,124 individuals who came for HCV antibody screening on their own initiative or following medical instruction. In addition, 99 HCV-positive patients receiving care at CERBA/LABIOGENE were recruited. Viral load and genotyping were performed in all the HCV identified positives cases.
Who Is Most At Risk Of Contracting Hepatitis C
You have a high risk of contracting hepatitis C if you:
- use or have used injection drugs even if it was just once or many years ago
- have received blood or blood products or an organ transplant before July 1990 in Canada
- have been in jail or
- have been injected or scratched during vaccination, surgery, blood transfusion or a religious/ceremonial ritual in regions where hepatitis C is common.
You have a high moderate risk of contracting hepatitis C if you:
- have tattoos or body piercing
- have multiple sexual partners
- have a sexually transmitted infection , including HIV or lymphogranuloma venereum
- have experienced traumatic sex or rough sex or have used sex toys or fisting that can tear body tissue
- have vaginal sex during menstruation
- have received a kidney treatment
- have received an accidental injury from a needle or syringe
- have another infectious disease
- were born to a hepatitis C infected mother or
- have a sexual partner infected with hepatitis C.
Hepatitis C is NOT passed from person to person by:
- coughing, sneezing
- breastfeeding unless your nipples are cracked and bleeding or
- oral sex, unless blood is present.
Read Also: How Do They Check For Hepatitis C
How Can I Cover Medication Costs
New therapies called direct-acting antivirals are effective and can achieve cures of over 90%. Because these new therapies are very new, they remain very expensive. As such, drug coverage from both government and private companies may require that your liver disease has progressed to a certain stage before they are willing to cover the cost of these drugs.
Talk with your healthcare provider about financial support that may be available.
Below are useful resources when looking for financial assistance:Private health insurance or drug plansIf you have private health insurance or a drug plan at work, you may be able to have the medication paid through your plan. Please consult your private health insurance or drug plan provider to see if your drug is covered.
Publicly funded plansEach provincial and territorial government offers a drug benefit plan for eligible groups. Some are income-based universal programs. Most have specific programs for population groups that may require more enhanced coverage for high drug costs. These groups include seniors, recipients of social assistance, and individuals with diseases or conditions that are associated with high drug costs. For more details, please contact your provincial or territorial health care ministry, or click on the appropriate link below.
Yukon
Available Patient Assistance Programs for Hepatitis C treatment Holkira Pak Maviret
MerckCare Hepatitis C Program 1 872-5773 Zepatier
Your Viral Load Doesn’t Affect Symptoms
Symptoms can develop within two weeks of your exposure to the virus. But, many people with a hepatitis C viral load have no symptoms. Possible signs of a hepatitis C infection include , , itchy skin, , , and . You also might have sore muscles, stomach pain, , and yellowing of the skin and eyes. Still, some people live for decades with hepatitis C and have none of these symptoms.
Recommended Reading: Do You Die From Hepatitis C
You Might Test Positive For Hepatitis C With An Undetectable Viral Load
Even if your viral load is too low to show up on a blood test, you could still have hepatitis C antibodies in your blood. Antibodies are the signs that you were exposed to the virus. Your body released these chemicals to fight the infection. And, even if the virus is gone, the antibodies stay in your blood. That’s why a basic hepatitis C test could still give you “positive” results. It’s also another reason that the viral load blood test is so important.
Different Types Of Viral Load Testing
There are two approaches to measuring viral load: quantitative testing and qualitative testing. Quantitative testing uses different techniques to measure viral load, including polymerase chain reaction , branched-chain DNA , and transcription-mediated amplification . Both PCR and TMA measure HCV RNA levels in the blood and are accurate down to 10-15 IU/mL. The bDNA is less complex than PCR and TMI, and is limited to measurement of viral loads above 615 IU/mL.1
Quantitative testing is typically used before starting treatment to determine a baseline viral load for the purpose of evaluating treatment response. Qualitative testing is capable of detecting low levels of HCV RNA. It gives results as positive or negative and, in some cases, has a lower limit of detection below 10 IU/mL. Qualitative testing is typically used to confirm diagnosis, where a specific viral count is not needed.1
You May Like: Hepatitis C Causes Symptoms And Treatment
Patient Characteristics And Viral Subtypes
There were no significant differences in sex or age between patients infected with the three types . The grading score for genotype 1b patients was significantly higher than that for genotype 2a patients due to the apparently higher score for W type patients than that for J type patients . No difference in staging score was detected between genotype 2a and 1b patients, or between subtype W and J patients. Patients with a blood transfusion history were more closely associated with genotype 1b, irrespective of subtype . Forty four of the total were complete responders of 79 genotype 1b patients and 24 of 42 genotype 2a patients). Genotype 2a patients had lower viral loads and responded to IFN better than 1b patients. W and J type patients showed similar viral loads and IFN responsiveness. The prevalence of the ISDR mutation was also similar between W and J type patients.