Lateral Flow Rapid Diagnostic Tests
Three HBV immunochromatographic RDT that have been prequalified by the World Health Organization include Determine HBsAg , VIKIA HBsAg and SD Bioline WB . These RDTs have met WHO ASSURED criteria and detect HBsAg and HBeAg in a single-use, disposable format . Evaluation of a rapid immunochromatographic test for simultaneous detection of HBsAg and HBeAg, in comparison with standard EIA, showed excellent accuracy, with sensitivity and specificity for HBsAg detection at 95 and 100%, respectively, although performance characteristics for HBeAg detection were decreased compared with the EIA method .
Several immunochromatographic lateral flow RDT have been tested and found suitable for HCV diagnosis. Mahajan et al. , compared three different RDT manufactured in India for detection of HCV antibody. They found moderate sensitivity and excellent specificity with HCV suspected cases within a Delhi liver clinic, with increased sensitivity among RNA positive patients . The InTec POC cassette has been evaluated in several HCV screening studies and displays good sensitivity and specificity for screening general and high-risk populations in Pakistan . Sensitivity of HCV RDT among HIV/HCV co-infected populations can be affected by HIV status. Hence, external validation of the RDT is needed prior to testing in such populations .
Assessment Of Hbv Immune Status
Immunity to HBV is acquired from a resolved infection or from vaccination .2). The HBV vaccine has been shown to induce protective immunity in 90% to 95% of vaccinees. Most vaccinees will have protective levels of anti-HBs for five to 10 years after vaccination, although the exact duration of immunity remains undefined. When anti-HBs levels have waned below the protective threshold of 10 mIU/mL, a booster dose of HBV vaccine has been shown to induce a strong anamnestic immune response in such individuals. It is therefore probable that protection from chronic HBV infection may last for decades and may well be lifelong .
Investigation of hepatitis B virus immunity. Anti-HBc-Total Total antibody to hepatitis B core protein Anti-HBs/HBsAb Antibody to hepatitis B surface antigen
The HBV immune status can be determined using the tests outlined below, but testing for vaccine immunity in the general population is not indicated unless the individual is at high risk of infection .3). Nonimmune individuals should be offered HBV vaccination where clinically appropriate.
Diagnosing Hepatitis A B & C
At NYU Langone, hepatologists, or liver specialists, and infectious disease specialists use blood tests to diagnose hepatitis A, B, and C. These viral infections cause inflammation of the liver.
If the results of a blood test confirm a diagnosis of viral hepatitis, your doctor may recommend imaging tests or a liver biopsy to determine the extent of liver disease.
You May Like: Can You Get Hepatitis From Saliva
Why It Is Done
Hepatitis B testing is done to:
- Find the type of infection and see if an infection has occurred recently or in the past.
- Screen people who have a higher chance of getting or spreading hepatitis B. This includes doctors, dentists, and nurses.
- Screen blood donors and donor organs to prevent the spread of hepatitis B.
- Find out if a person has antibodies after getting a hepatitis B vaccination. Having antibodies means the vaccine worked.
- Find out if hepatitis B is the cause of abnormal liver function tests.
- See how well treatment of chronic hepatitis B is working.
What Are The Treatments For Viral Hepatitis
The treatment for viral hepatitis depends on the type and stage of the infection. Over the last several years, excellent treatments for both hepatitis B and C have become available. More and improved treatments are being evaluated all the time.
Your primary care doctor should be able to provide adequate care of your hepatitis. However, if you have severe hepatitis, you may require treatment by a hepatologist or gastroenterologist — specialists in diseases of the liver. Hospitalization is normally unnecessary unless you cannot eat or drink or are vomiting.
Hepatitis A usually requires minimal treatment and your liver usually heals within 2 months. Make sure you stay hydrated and well-nourished. While a vaccination can prevent you from getting hepatitis A, once you have had it, you cannot be re-infected.
Doctors sometimes recommend drug therapy for people with hepatitis B and C. Antiviral medication for hepatitis B includes adefovir , entecavir , interferon, lamivudine , peginterferon , telbivudine , and tenofovir.
Until recently, the standard treatment for chronic hepatitis C was a course of peginterferon plus ribavirin for people with genotype 2 and 3, and peginterferon plus ribavirin plus a protease inhibitor for people with genotype 1. These treatments had been shown to be effective in from 50% to 80% of those infected with hepatitis C but the side effects were very difficult for people to tolerate.
Hepatitis in Pregnant Women
Other Points to Consider
Show Sources
Recommended Reading: Hepatitis B What Is It
When Should I Get Hepatitis B Testing
Using hepatitis B tests to screen for HBV is recommended for certain groups that are at an increased risk of infection. Groups that may benefit from hepatitis B screening include:
- Pregnant people
- People born in parts of the world where hepatitis B is more common, including Africa, Asia, Eastern Europe, South America, and parts of the Middle East
- People who didnât receive a hepatitis B vaccine
- HIV-positive people
- Pain in the joints or abdomen
- Loss of appetite, nausea, or vomiting
- Yellowish skin and eyes
Using hepatitis B testing to assess immunity to HBV may be used before or after vaccination. Pre-vaccination testing is not always needed but may be performed if there is a chance that a patient has previously been infected with HBV or has already been vaccinated. Post-vaccination testing is used in certain groups of people who are at an especially elevated risk for HBV infection, including infants born to mothers with a hepatitis B infection.
Molecular Methods For Hbv Infection
HBV DNA is a direct measurement of the viral load, which reveals the replication activity of the virus. It is detectable at the early stage of infection and increases up to peak level approximately 3 months after the exposure to HBV and then gradually diminishes in chronic infection or disappears at the recovery from HBV infection.
As the prevalence of serologically negative HBV infection has increased, HBV-DNA detection has obtained more awareness in clinical medicine . The detection of HBV DNA is a reliable marker of replication activity, and higher titers of HBV DNA are related to the more rapid disease progression and higher incidence of HCC . Furthermore, HBV DNA testing is useful in routine clinical setting to determine patients who need antiviral therapy and monitor them for suitable treatment .
There are two principles of techniques to identify and quantify HBV DNA: signal amplification such as hybrid capture and branched DNA technology target amplification such as polymerase chain reaction . Real-time PCR can detect wide dynamic range of viral load . For this reason, it has come to be the standard method to detect and quantify HBV DNA in clinical setting. Furthermore, it can be fully automated and does not generate carry-over contamination . Table 1 displays the comparison of assays for quantitative measurement of HBV DNA.
Table 1
Recommended Reading: How To Contract Hepatitis B And C
What If I Have Symptoms Of Viral Hepatitis
If you have symptoms or signs of viral hepatitis, your health care provider can perform a blood test to check for the presence of an antibody. If you have hepatitis B or C, more blood samples may be necessary later — even if the symptoms have vanished — to check for complications and determine if you have progressed from acute to chronic disease. Most people have vague or no symptoms at all hence, viral hepatitis is often referred to as a silent disease.
Your healthcare provider may also require a liverbiopsy, or tissue sample, in order to determine the extent of the damage. A biopsy is commonly performed by inserting a needle into the liver and drawing out a fragment of tissue, which is then sent to a lab to be analyzed.
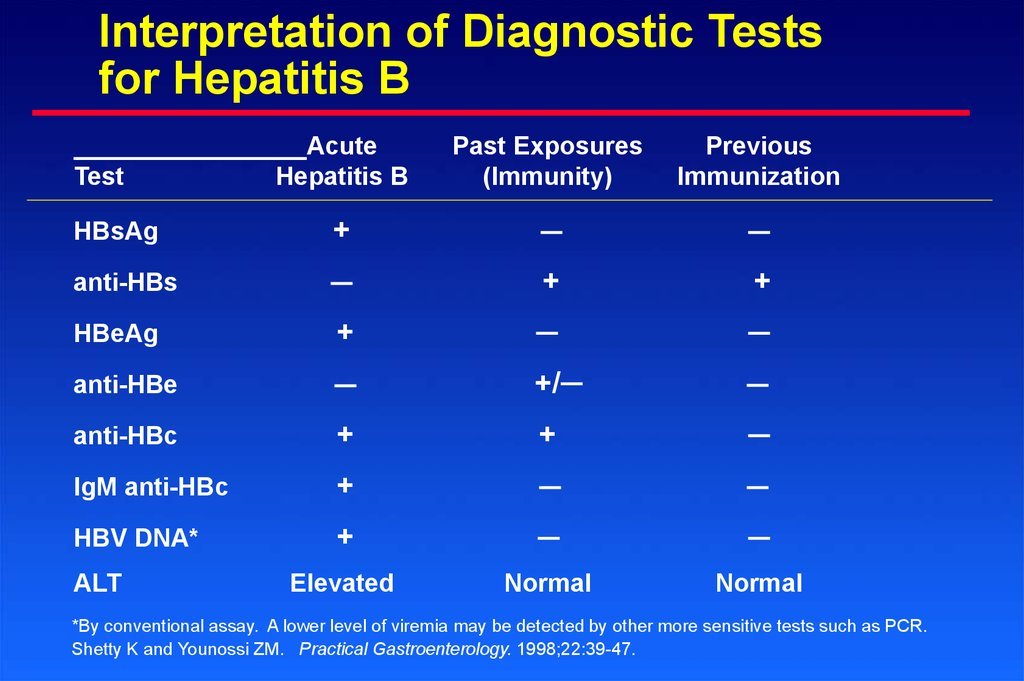
Interpretation Of Hbv Serologic Tests
The three major tests used for hepatitis B screening are HBsAg, anti-HBs, and anti-HBc. The following summarizes the interpretation of test results with these three serologic tests . The anti-HBc test may consist of a total anti-HBc or an IgM anti-HBc. The IgM anti-HBc has value primarily when considering acute hepatitis B infection. For diagnostic purposes, testing for HBeAg and anti-HBe is usually not performed, since they typically do not provide additional diagnostic information. For persons who are diagnosed with HBV infection, evaluation of HBeAg, anti-HBe, and HBV DNA are all usually performed and monitoring of these labs can be important in persons on treatment for chronic HBV.
- Immune from Vaccination: All modern HBV vaccines utilize recombinant HBsAg as the primary immunogen. The host serologic response to the vaccine is the development of anti-HBs. The hepatitis B vaccines do not generate an anti-HBc immune response. A positive anti-HBs in conjunction with negative HBsAg and negative anti-HBc indicates immunity as a result of vaccination, whereas a positive titer for both anti-HBs and anti-HBc indicates immunity from past infection with HBV. An anti-HBs titer greater than 10 to 12 mIU/mL correlates with protective immunity.
Also Check: Does Hepatitis C Cause Itching
Diagnostic Accuracy Compared To A Nucleic Acid Reference Standard
Rapid diagnostic tests
Three studies evaluated 7 RDTs in samples from 510 patients against a NAT reference standard, although some samples were used for multiple testing episodes with different tests. One study used plasma from Nigerian repeat blood donors. Sensitivities ranged from 38% to 99% and specificities ranged from 94 to 99%. Overall pooled sensitivity and specificity were 93.3% and 98.1% , respectively, with significant heterogeneity in terms of sensitivity . One case-control study evaluating five different tests in 240 Iranian patients, had significantly higher sensitivity and specificity compared to the other studies, contributing to the overall statistical heterogeneity .
Fig. 9
One study assessed RDT performance in 113 HIV-negative Nigerian repeat blood donors, with clinical sensitivity 60% of note the 8 false negative samples were anti-HBc-positive and regarded as occult hepatitis B, with median HBV viral load 51 IU/ml . The final study had data for consecutive HIV-positive and negative individuals in Uganda sensitivity was lower in the 83 HIV-positive patients compared to the 74 HIV-negative individuals at 38% and 55% respectively .
Enzyme immunoassays
Five studies evaluated EIAs based on a NAT reference, using samples from 1194 patients. Pooled sensitivity and specificity were 75.7% and 86.1% , respectively. The respective positive and negative LRs were 7.2 and 0.30 , with reduced heterogeneity compared to studies evaluating RDTs .
Fig. 10
Treatment Indications And Phases Of Chronic Hbv Infection
Phases of Chronic HBV Infection
| Phase | |
|---|---|
| HBV DNA | Inflammation |
|
May or may not be indicated |
|
|
Immune tolerant |
HBV = hepatitis B virus + = detectable = undetectable +/= may or may not be detectable.
Information from reference 22.
Phases of Chronic HBV Infection
| Phase | |
|---|---|
| HBV DNA | Inflammation |
|
May or may not be indicated |
|
|
Immune tolerant |
HBV = hepatitis B virus + = detectable = undetectable +/= may or may not be detectable.
Information from reference 22.
Also Check: How Does Hepatitis C Affect The Liver
Assessment Of The Quality Of The Studies
The QUADAS-2 assessment for risk of bias of each study, including sub-studies deriving separate data points is presented in , with a summary in . Bias in patient selection was generally attributable to a case-control study design , or from enrolment of highly selected populations such as blood donors or those with known hepatitis B virus infection. Risk of bias from the index test was most commonly due to insufficient reporting of blinding or evaluation of RDTs which are no longer commercially available. Although the majority of studies did not specify the exact time interval between performance of the index and reference assays, it was assumed to be at low risk of bias as the assays were performed on the same sample. Applicability was judged to be higher risk for bias predominantly due to inclusion of older studies, those that evaluated tests which are no longer commercially available or studies using a NAT reference.
Fig. 2
Diagnosis Of Hepatitis B

Jeong Eun Song, Do Young Kim
Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
Contributions: Conception and design: DY Kim Administrative support: None Provision of study materials or patients: None Collection and assembly of data: None Data analysis and interpretation: None Manuscript writing: All authors Final approval of manuscript: All authors.
Correspondence to:
Abstract: Hepatitis B virus infection is a major global health problems leading to severe liver disease such as cirrhosis and hepatocellular carcinoma . HBV is a circular, partly double-stranded DNA virus with various serological markers: hepatitis B surface antigen and anti-HBs, anti-HBc IgM and IgG, and hepatitis B e antigen and anti-HBe. It is transmitted by sexual, parenteral and vertical route. One significant method to diminish the burden of this disease is timely diagnosis of acute, chronic and occult cases of HBV. First step of HBV diagnosis is achieved by using serological markers for detecting antigens and antibodies. In order to verify first step of diagnosis, to quantify viral load and to identify genotypes, quantitative or qualitative molecular tests are used. In this article, the serological and molecular tests for diagnosis of HBV infection will be reviewed.
Keywords: Hepatitis B virus serology molecular diagnosis
Submitted Aug 01, 2016. Accepted for publication Aug 28, 2016.
doi: 10.21037/atm.2016.09.11
Recommended Reading: Cost For Hepatitis B Test
Specimen Choice Collection And Transport
The specimen of choice for the diagnosis of HBV infection is blood. Serological tests for viral antigens and antibodies are typically used for diagnostic screening and can be performed on either serum or plasma. Both HBV antigens and antibody are stable at room temperature for days, at 4°C for months, and frozen at -20°C to -70°C for many years. Because modern testing involves automated enzyme immunoassays that depend on colourimetic or chemiluminescence signal measurement, care should be taken to avoid hemolysis of the sample because it may interfere with the ability of the assay to accurately detect these markers.
A number of nucleic acid-based tests, which have been the subject of recent reviews , are available to directly detect HBV-DNA in serum or plasma. Care must be taken to avoid the degradation of the viral nucleic acid in the specimen, which can result in falsely low or no measurable viral load. Serum should therefore be removed from clotted blood within 4 h of collection and stored at -20°C to -70°C , and can be subjected to up to eight short-term freeze-thaw cycles without significant loss of detectable HBV-DNA . Alternatively, the presence of EDTA in plasma is known to stabilize viral nucleic acids. EDTA blood can be stored for up to five days at 4°C without affecting the viral load . Polymerase chain reaction-based tests can use either serum or plasma, while hybridization-based assays recommend the use of serum.
Study Selection And Characteristics
A total of 11,589 citations were identified, and 293 full-text articles examined which identified 40 studies meeting pre-defined criteria . Of the included studies, 33 compared RDTs and/or EIAs against an immunoassay reference standard, of which five focused on accuracy in HIV-positive individuals . Seven studies compared RDTs and/or EIAs against a NAT reference standard, of which 3 had data from HIV-positive patients . Studies were all either cross-sectional or case-control, predominantly in the laboratory setting, and performed in a broad range of populations, including healthy volunteers, blood donors, pregnant women, incarcerated adults, HIV and hepatitis patient cohorts with confirmed HBV infection. The prevalence of HBV ranged from 1.9 to 84% in populations tested. A mixture of serum, plasma and whole blood was used for RDTs, while studies of EIAs were performed on serum or plasma samples. Study characteristics are presented in Tables , and .
Fig. 1
Don’t Miss: Can You Donate Plasma If You Had Hepatitis B
Recommended Tests To Investigate Chronic Hbv Infection And The Interpretation Of Results
Chronic HBV infection is defined by the continued presence of HBsAg in the blood for longer than six months. Figure and Table outline the tests used to diagnose most cases of chronic HBV. Test selection should be based on the person’s risk factors, vaccination history and findings from previous tests .
Diagnostic tests for acute or chronic hepatitis B virus infection . ALT Alanine aminotransferase Anti-HAV-IgM Immunoglobulin M class antibody to HAV Anti-HCV Antibody to HCV antigens HBsAg Hepatitis B surface antigen
Diagnostic Accuracy Of Laboratory Immunoassays For Hbsag Detection
Five studies , performed in China, Ghana, Cambodia and Vietnam evaluated 8 EIAs against a CMIA reference standard, in 1825 serum or plasma samples, reported a pooled sensitivity and specificity of 88.9% and 98.4% , respectively. The respective positive and negative LRs were 46.8 and 0.04 , with visible and statistical heterogeneity between studies . Outliers were from two Chinese studies that evaluated two older ELISA assays with a sensitivity lower than 90% .
Fig. 8
One study evaluated 3 different EIAs in 838 HIV-positive patients. Results were homogenous between tests, with pooled sensitivity and specificity of 97.9% and 99.4% , respectively, for a positive and negative LR of 167.3 and 0.02 respectively .
Also Check: Medicine For Hepatitis C Virus
Antibody To Hcv Antigens
-
If negative, chronic HCV infection is ruled out in immunocompetent individuals. Because the antibody to HCV antigens response in immunocompromised persons can be blunted, a qualitative test for HCV-RNA may be required to rule out occult infection in such individuals. A new HCV core antigen test, which is currently under evaluation, can also be used to confirm active infection . However, the currently available HCV core antigen test is less sensitive, detecting less than 90% to 95% of HCV-RNA-positive specimens . Therefore, the current HCV core antigen should not be used for definitive exclusion of active infection.
-
If found to be anti-HCV positive, the patient has been infected with HCV. Because most HCV infections are chronic , the presence of anti-HCV is correlated with active infection however, a qualitative test for HCV-RNA is currently required to confirm active HCV infection .