Hbv Dna Hbv Genotype And Hbv Drug Resistance Assays
Specimen: Serum or plasma
Container: Red-top tube, yellow-top tube , gel-barrier tube, plasma preparation tube, or lavender tube
Collection method: Routine venipuncture
The specimen should be transfused to separate plasma/serum from cells within 6 hours and kept frozen when testing cannot be done promptly.
The tests use PCR amplification, DNA probe hybridization, and sequencing method.
Assessment Of Hbv Immune Status
Immunity to HBV is acquired from a resolved infection or from vaccination .2). The HBV vaccine has been shown to induce protective immunity in 90% to 95% of vaccinees. Most vaccinees will have protective levels of anti-HBs for five to 10 years after vaccination, although the exact duration of immunity remains undefined. When anti-HBs levels have waned below the protective threshold of 10 mIU/mL, a booster dose of HBV vaccine has been shown to induce a strong anamnestic immune response in such individuals. It is therefore probable that protection from chronic HBV infection may last for decades and may well be lifelong .
Investigation of hepatitis B virus immunity. Anti-HBc-Total Total antibody to hepatitis B core protein Anti-HBs/HBsAb Antibody to hepatitis B surface antigen
The HBV immune status can be determined using the tests outlined below, but testing for vaccine immunity in the general population is not indicated unless the individual is at high risk of infection .3). Nonimmune individuals should be offered HBV vaccination where clinically appropriate.
How To Get Tested
Hepatitis B testing is typically prescribed by a doctor and performed in a hospital, lab, or other medical setting. Taking a hepatitis B test requires a blood sample, which can be collected by a health care professional.
For laboratory-based testing, blood is drawn from a patientâs vein. After blood is collected, the sample is sent to a laboratory for analysis.
You May Like: Hepatitis B Liver Cancer Symptoms
Transmission Symptoms And Treatment
How is HBV transmitted?
HBV is transmitted through activities that involve percutaneous or mucosal contact with infectious blood or body fluids , including
- sex with an infected partner
- injection-drug use that involves sharing needles, syringes, or drug-preparation equipment
- birth to an infected mother
- contact with blood from or open sores on an infected person
- exposures to needle sticks or sharp instruments and
- sharing certain items with an infected person that can break the skin or mucous membranes , potentially resulting in exposure to blood.
How long does HBV survive outside the body?
HBV can survive outside the body and remains infectious for at least 7 days .
What should be used to clean environmental surfaces potentially contaminated with HBV?
Any blood spills should be disinfected using a 1:10 dilution of one part household bleach to 10 parts of water. Gloves should be worn when cleaning up any blood spills.
Who is at risk for HBV infection?
The following populations are at increased risk for becoming infected with HBV:
- Infants born to infected mothers
- Sex partners of infected people
- Men who have sex with men
- People who inject drugs
- Household contacts or sexual partners of known people with chronic HBV infection
- Health-care and public-safety workers at risk for occupational exposure to blood or blood-contaminated body fluids
- Hemodialysis patients
Who should be screened for HBV?
CDC recommends that the following people be screened for HBV :
- fever,
When And How To Perform Post
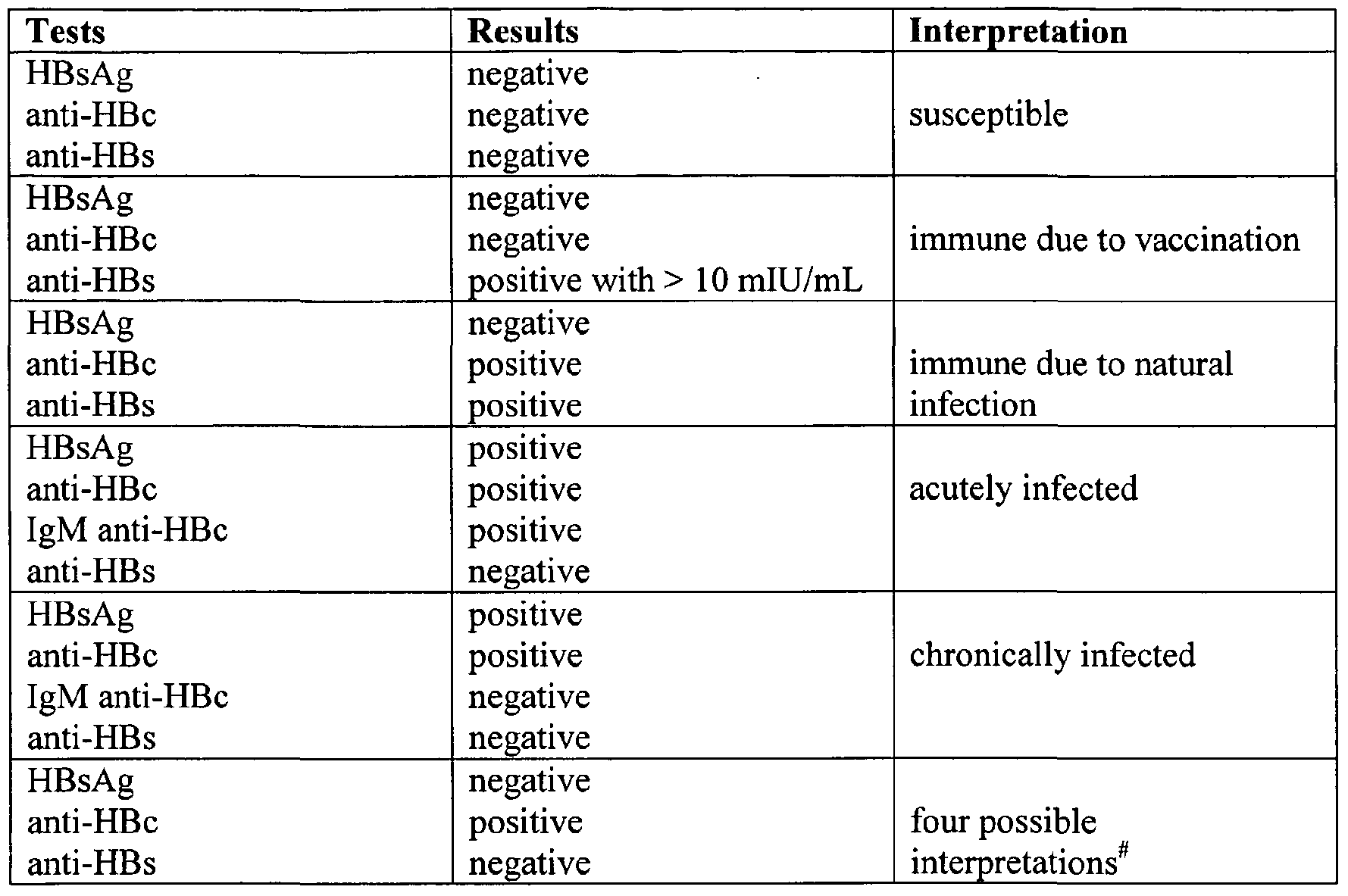
Which test to use: If testing is needed following vaccination, use quantitated HBsAb only
- Post-vaccination testing is needed for certain groups who are at especially high risk for HBV infection
- The purpose of post-vaccination testing is to confirm if patients have achieved adequate immune response as measured by hepatitis B surface antibody
- Perform testing 1-2 months after final dose of the HBV vaccine series
- Persons with HBsAb concentrations of > 10 mIU/ml are considered immune
- Post-vaccination testing is recommended for some patients:
- Infants born to HBsAg+ women
- Infants born to women whose HBSAg status remains unknown
- Health care personnel and public safety workers at risk for blood or body fluid exposure
- Hemodialysis patients
- Other immunocompromised persons such as hematopoietic stem-cell transplant patients or persons receiving chemotherapy
- Sex partners of HBSAg+ persons
Don’t Miss: How Much Does A Hepatitis A Shot Cost
How Much Does The Test Cost
The cost of hepatitis B testing depends on the tests that are performed, where the test is conducted, and a patientâs health insurance coverage. When testing is ordered by a doctor, patients with health insurance may find it helpful to discuss the cost of testing with their health insurance company as they may be responsible for testing costs as well as other out-of-pocket costs such as copays and deductibles.
For patients without health insurance or for whom insurance doesnât cover the cost of testing, it may be helpful to discuss the cost of hepatitis B testing with a doctor or hospital administrator.
The cost of at-home hepatitis B testing starts around $45. At-home test kits may also test for additional types of viral hepatitis in the same sample. The cost of test panels that look for more than one type of viral hepatitis start around $80.
How Is Hepatitis B Treated
There is no specific treatment for acute hepatitis B infections. Symptoms are usually treated with supportive care. This usually involves making sure that you are getting plenty of rest and enough fluids and nutrition by eating and drinking small amounts several times a day.
Chronic forms of hepatitis B may be treated with antiviral medications such as interferon, entecavir, tenofovir, lamivudine, and adefovir. However, some antiviral drugs can have serious side effects and not all people need to be treated. Often, people with chronic hepatitis will be closely monitored to see if they develop cirrhosis or liver cancer. It is important to talk to your healthcare provider about your treatment options and the risks and benefits of those currently available.
Don’t Miss: Hepatitis C Cdc Fact Sheet
Chicken Pox Immunity Screen
Chickenpox is a disease caused by the varicella-zoster virus. It is highly contagious and is most often characterized by a red rash that covers the body within a few weeks of infection. Because of the introduction of a chickenpox vaccine, the disease is now considered rare. For information on vaccination, contact your medical provider or visit the CDC or Arizona Department of Health Services websites.
What Is The Difference Between Hepatitis B Surface Antibody And Antigen
An antigen is a substance that induces antibody production. Hepatitis B surface antigen is a protein on the surface of hepatitis B virus.
Hepatitis B surface antibodies are produced by the bodys immune system in response to HBsAg. The presence of adequate hepatitis B surface antibodies in the blood indicates protection against hepatitis B virus infection.
Don’t Miss: What Is Hepatitis B Surface Antibody
Question 3 How Is The Quantitative Hepatitis B Surface Antibody Test Performed
An immunometric technique is used. The anti-HBs binds to HBsAg ad and ay subtypes, which are coated on the test wells. Binding of a horseradish peroxidase-labeled HBsAg conjugate to the anti-HBs completes the sandwich formation. Unbound materials are then washed away. In the next step, the horseradish peroxidase catalyzes oxidation of a luminogenic substrate, producing light. Light signals are detected and quantified. Intensity of the light is proportional to the amount of anti-HBs present in the patient sample. The result is standardized to an international unit system and reported as milliinternational units per milliliter .
Clinical Information Discusses Physiology Pathophysiology And General Clinical Aspects As They Relate To A Laboratory Test
Hepatitis B e antigen is a small polypeptide that exists in a free form in the serum of individuals during the early phase of hepatitis B infection, soon after hepatitis B surface antigen becomes detectable. Serum levels of both HBeAg and HBsAg rise rapidly during the period of viral replication. The presence of HBeAg in serum correlates with hepatitis B virus infectivity, the number of infectious virions, and the presence of HBV core antigen in the infected hepatocytes.
During recovery from acute hepatitis B, HBeAg level declines and becomes undetectable in the serum, while hepatitis B e antibody appears and becomes detectable in the serum. Anti-HBe usually remains detectable for many years after recovery from acute HBV infection.
In HBV carriers and patients with chronic hepatitis B, positive HBeAg results usually indicate presence of active HBV replication and high infectivity. A negative HBeAg result indicates very minimal or no HBV replication. Positive anti-HBe results usually indicate inactivity of the virus and low infectivity. Positive anti-HBe results in the presence of detectable HBV DNA in serum also indicate active viral replication in these patients.
Read Also: Hepatitis C Ab Non Reactive
Specimen Choice Collection And Transport
The specimen of choice for the diagnosis of HBV infection is blood. Serological tests for viral antigens and antibodies are typically used for diagnostic screening and can be performed on either serum or plasma. Both HBV antigens and antibody are stable at room temperature for days, at 4°C for months, and frozen at -20°C to -70°C for many years. Because modern testing involves automated enzyme immunoassays that depend on colourimetic or chemiluminescence signal measurement, care should be taken to avoid hemolysis of the sample because it may interfere with the ability of the assay to accurately detect these markers.
A number of nucleic acid-based tests, which have been the subject of recent reviews , are available to directly detect HBV-DNA in serum or plasma. Care must be taken to avoid the degradation of the viral nucleic acid in the specimen, which can result in falsely low or no measurable viral load. Serum should therefore be removed from clotted blood within 4 h of collection and stored at -20°C to -70°C , and can be subjected to up to eight short-term freeze-thaw cycles without significant loss of detectable HBV-DNA . Alternatively, the presence of EDTA in plasma is known to stabilize viral nucleic acids. EDTA blood can be stored for up to five days at 4°C without affecting the viral load . Polymerase chain reaction-based tests can use either serum or plasma, while hybridization-based assays recommend the use of serum.
Recommended Tests To Investigate Acute Hbv Infection And The Interpretation Of Results
Acute HBV infection generally presents after an incubation period of six weeks to several months with an onset of nonspecific symptoms that may include fever, malaise, anorexia and nausea, followed by the onset of jaundice, dark urine and pale stools. Approximately 25% to 40% of infected adults will be symptomatic, and most will demonstrate elevations in ALT however, infants, toddlers and immunosuppressed individuals may not manifest signs or symptoms of infection. The management of acute infections is largely supportive unless fulminant hepatitis develops, in which case the patient should be referred to a liver specialist. Because the clinical features of acute hepatitis are very similar for HAV, HBV and HCV, testing for all three agents should be performed when working up an acute case. While the sexual transmission of HCV is rare, varying between zero to six cases per 1000 person-years , HAV that is typically spread by the fecal-oral route poses a clear risk to sexual partners. Figure and Table outline the appropriate serological tests to investigate acute hepatitis.
Read Also: How Is Hepatitis A Spread
What Does The Test Result Mean
The tests for hepatitis B may be ordered individually but are often ordered in some combination, depending on the reason for testing. Results of the tests are typically evaluated together. Sometimes the meaning of one result depends on the result of another test. However, not all tests are performed for all people.
The table below summarizes possible interpretations of some common patterns of results.
| Initial Tests | |
| None detected or detected at very low level | Chronic infection but low risk of liver damage carrier state |
*Note: There are some types of HBV that do not make e-antigen. In areas where these strains of HBV are common , testing for HBeAg is not very useful. In these cases, a negative HbeAg result does not necessarily mean that the person is not infectious it may be that the person is infected with a strain that does not make the e-antigen.
Monitoring treatment of chronic infection: If the results from initial and follow-up testing indicate that a person has chronic hepatitis B, then the individual may be treated with medication and the effectiveness of that treatment may be monitored using the tests for HBe and HBs antigen and antibody and HBV DNA:
Is There Anything Else I Should Know
Even if you don’t have symptoms, an HBV infection can damage your liver and you can spread the infection to others. For this reason, it is important to get tested if you think you have been exposed to HBV.
Blood banks screen all donated blood for the hepatitis B virus , hepatitis B surface antigen , and hepatitis B core antibody . Donors are notified of any confirmed positive reactions. People who receive a notice regarding possible infection with hepatitis B after donating should visit their healthcare provider for further testing. The healthcare practitioner will order additional tests to make a proper diagnosis and determine if treatment is necessary.
If exposed to HBV and you haven’t been vaccinated, an infection can be avoided by getting a shot of hepatitis B immune globulin within 24 hours and typically you will also be given the first dose of the hepatitis B vaccine.
A test is available to determine the specific type of hepatitis B virus that is causing a person’s infection. This is called HBV genotyping. However, this testing is currently mainly used in research settings and not for clinical purposes.
You May Like: Where Can I Get The Hepatitis B Vaccine For Free
Hepatitis B Surface Antibody Qualitative
Test Code: 499
Methodology: Immunoassay
Clinical Significance: The detection of anti-HBs is indicative of a prior immunologic exposure to the antigen or vaccine. To determine immune status as 10 mIU/mL as per CDC guidelines, please order Hepatitis B Surface Antibody, Quantitative.
Alternative Name: Anti-HBS Anti-HBS Qual Anti-HBSAG Australian Antibody HB Surface Ab
Supply: T01 – Red/Gray SST 8.5mL
Preferred Specimen: Serum
Transport Container: Serum Separator Tube
Transport Temperature: Room Temperature
Specimen Stability: Room Temperature: 5 days
Rejection Criteria: Gross Hemolysis, Gross Lipemia
For additional supply or collection device information, please contact DLO’s Customer Service at 891-2917, option 2.
The information contained here on the Diagnostic Laboratory of Oklahoma website is not to be construed as medical recommendations or professional advice. Neither DLO nor its affiliates, agents or any other party involved in the preparation or publication of the works presented is responsible for any errors or omissions in information from the use of such information. Readers are encouraged to confirm the information contained herein with other reliable sources and to direct any questions concerning personal health care to licensed physicians or other appropriate health care professionals.
Detection Of Antiviral Resistance
Lamivudine monotherapy has been reported to be associated with the rapid emergence of antiviral resistance in 15% to 60% of treated individuals . Resistant HBV genomes have mutations in codon 552 within the YMDD motif of the reverse transcriptase/polymerase where a valine or isoleucine replaces the methionine. Resistance is typically clinically manifested by significant elevations in ALT after an initial decline in response to treatment. Prolonged treatment after development of the YMDD mutant is controversial, although improvement in liver pathology with decreased fibrosis may occur with continuation of treatment. Concerns about disease flares after stopping lamivudine have been raised . The development of genotypic resistance can be documented by molecular sequencing or by the INNO-LiPA HBV DR assay , which involves hybridization of amplified HBV-DNA fragments onto specific nucleotide probes that have been immobilized on nitrocellulose strips .
Don’t Miss: Sign Symptoms Of Hepatitis B
Evaluation Of Individuals Suspected Of Having An Hbv Infection
Given the perinatal and childhood vaccination programs already in place in North America, most HBV-infected individuals will likely present with chronic infection. Such individuals are likely to have risk factors that include immigration from high endemicity regions, injection drug use or sexual contact with an infected person 1) . Therefore, the present guideline will provide diagnostic recommendations first for individuals suspected of having chronic HBV infection and, subsequently, for those with acute infection. The diagnosis of HBV infection in any individual has important management implications, including appropriate counselling, monitoring and/or treating and vaccinating family or at-risk contacts.
Question 7 Is Hepatitis B Surface Antibody Antibody Always Acquired After A Completed Vaccination Protocol
No. After 3 intramuscular doses of vaccine, > 90% of healthy adults and > 95% of those < 19 years of age develop immunity .1 However, there is an age-specific decline in development of immunity. After age 40 years, about 90% of people become immune, but by age 60 years, only 75% of people become immune.1 Larger vaccine doses or an increased number of doses are required to induce immunity in many hemodialysis patients and in other immunocompromised people.1
References
This FAQ is provided for informational purposes only and is not intended as medical advice. A clinicians test selection and interpretation, diagnosis, and patient management decisions should be based on his/her education, clinical expertise, and assessment of the patient.Document FAQS.105 Revision: 0
Also Check: Is Hepatitis B And Hiv The Same Thing
Taking A Hepatitis B Test
Testing for hepatitis B is performed on a sample of blood. A doctor, nurse, or other health care provider can obtain a blood sample using a small needle to draw blood from a vein.
At-home hepatitis B testing requires that users carefully follow instructions provided in the test kit to collect a small sample of blood, package the sample, and mail it to a lab for testing.
When To Get Tested

When you have risk factors for HBV infection or when you have signs and symptoms of hepatitis, such as jaundice or unexplained elevated blood levels of alanine aminotransferase , a liver-associated enzyme when you have a condition that requires chemotherapy or drugs that suppress your immune system when you are being treated for HBV or hepatitis C when it is unclear whether you have immunity and your healthcare practitioner is considering giving you the hepatitis B vaccine
Also Check: What Is A Hepatitis B Shot