Clinical Guidelines For Children With Chronic Hepatitis B
In general, the clinical guidelines for children are the same as for adults – visits are usually scheduled every six months or once a year. Most children do not need drug treatment, but they still need to be monitored regularly to make sure they remain healthy and to detect any problems with their liver as soon as possible. Visits will include a physical exam, blood tests, and possibly an imaging study of the liver .
AASLD guidelines provide guidance for treating children under the Updated Recommendations on the Treatment of Patients With Chronic Hepatitis B, section 9A.
The Hepatitis B Foundation convened the first Pediatric HBV Workshop and invited the nations leading pediatric liver specialists to develop the first national recommendations for children living with hepatitis B to ensure that they receive the best care possible. These recommendations have been published in highly respected, peer-reviewed journals and provide expert guidance for the care of infected children.
Hepatitis B Foundations Clinical Guidelines for Pediatric HBV
HBF’s Pediatric HBV Screening and Monitoring Recommendations Published in Pediatrics in November 2009Haber BA, Block JM, Jonas MM, Karpen SJ, London WT, McMahon BJ, Murray KF, Narkewicz MR, Rosenthal P, Schwarz KB. Recommendations for screening, monitoring, and referral of pediatric chronic hepatitis B. Pediatrics 124:e1007-13.
American Association For The Study Of Liver Diseases Recommendations
The 2016 AASLD guidelines for the treatment of chronic hepatitis B as well as select recommendations from the 2018 AASLD guidance update on the prevention, diagnosis, and treatment of chronic hepatitis B are outlined below and in the Guidelines section.
Adults with immune-active chronic hepatitis B infection
Administer antiviral therapy to lower the risk of morbidity and mortality associated with chronic hepatitis B infection.
The recommended initial agent for adults is PEG-IFN, entecavir, or tenofovir.
Adults with immune-tolerant chronic hepatitis B infection
Antiviral therapy is not recommended.
The AASLD suggests obtaining ALT levels at least every 6 months to monitor for potential transition to immune-active or -inactive chronic hepatitis B.
For select patients older than 40 years, the AASLD suggests antiviral therapy in the setting of normal ALT levels, elevated HBV DNA , and significant necroinflammation or fibrosis on liver biopsy specimens.
Adults with HBeAg-positive immune-active chronic hepatitis B who seroconvert to anti-HBe on nucleoside analog therapy
After a period of treatment consolidation , consider discontinuing NA therapy in noncirrhotic HBeAg-positive adults who seroconvert to anti-HBe while on NA treatment. If antiviral therapy is stopped, monitor the patient every 3 months for a minimum of 1 year for recurrent viremia, ALT flares, seroreversion, and clinical decompensation.
Adults with HBeAg-negative immune-active chronic HBV infection
Inpatient care
Screening For Viral Hepatitis
The purpose of screening for viral hepatitis is to identify people infected with the disease as early as possible, even before symptoms and transaminase elevations may be present. This allows for early treatment, which can both prevent disease progression and decrease the likelihood of transmission to others.
Hepatitis A
Hepatitis A causes an acute illness that does not progress to chronic liver disease. Therefore, the role of screening is to assess immune status in people who are at high risk of contracting the virus, as well as in people with known liver disease for whom hepatitis A infection could lead to liver failure. People in these groups who are not already immune can receive the hepatitis A vaccine.
Those at high risk and in need of screening include:
- People with poor sanitary habits such as not washing hands after using the restroom or changing diapers
- People who do not have access to clean water
- People in close contact with someone who has hepatitis A
- People who use illicit drugs
- People with liver disease
- People traveling to an area with endemic hepatitis A
The presence of anti-hepatitis A IgG in the blood indicates past infection with the virus or prior vaccination.
Hepatitis B
The CDC, WHO, USPSTF, and ACOG recommend routine hepatitis B screening for certain high-risk populations. Specifically, these populations include people who are:
Other
Hepatitis A
Hepatitis B and C
Hepatitis D
Hepatitis E
Alcoholic hepatitis
Don’t Miss: Hepatitis B How Do You Catch It
Ldv/sof Use In Children
There is preliminary data from 90 children treated with SOF/LDV 45 mg/200 mg, with or without ribavirin. Of the 90 children, there were 86, 3, and 2 with genotypes 1, 3, and 4 respectively 59% were male, 79% were whites, and 80% were treatment-naïve. Median age was 9 years, median weight was 33 kg, and median BMI was 18 kg/m2. Pharmacokinetic evaluation was done on day 10 in the first 12 participants, and found that LDV, SOF, and GS-331007 exposures were equivalent to those in adults. At the time of this review, 88/90 patients had completed therapy, and the rate of SVR at 4 weeks post-treatment in these 88 patients was 99% one genotype 1a patient relapsed at 12 weeks of LDV/SOF therapy. No patients experienced grade 3 or 4 adverse events, and the only serious adverse events reported were a tooth abscess, abdominal pain, and gastroenteritis not believed to be related to the study drug. The most common adverse events were abdominal pain, headache, diarrhea, vomiting, nausea, fatigue, fever, cough, and oropharyngeal pain.
National Institutes Of Health Recommendations
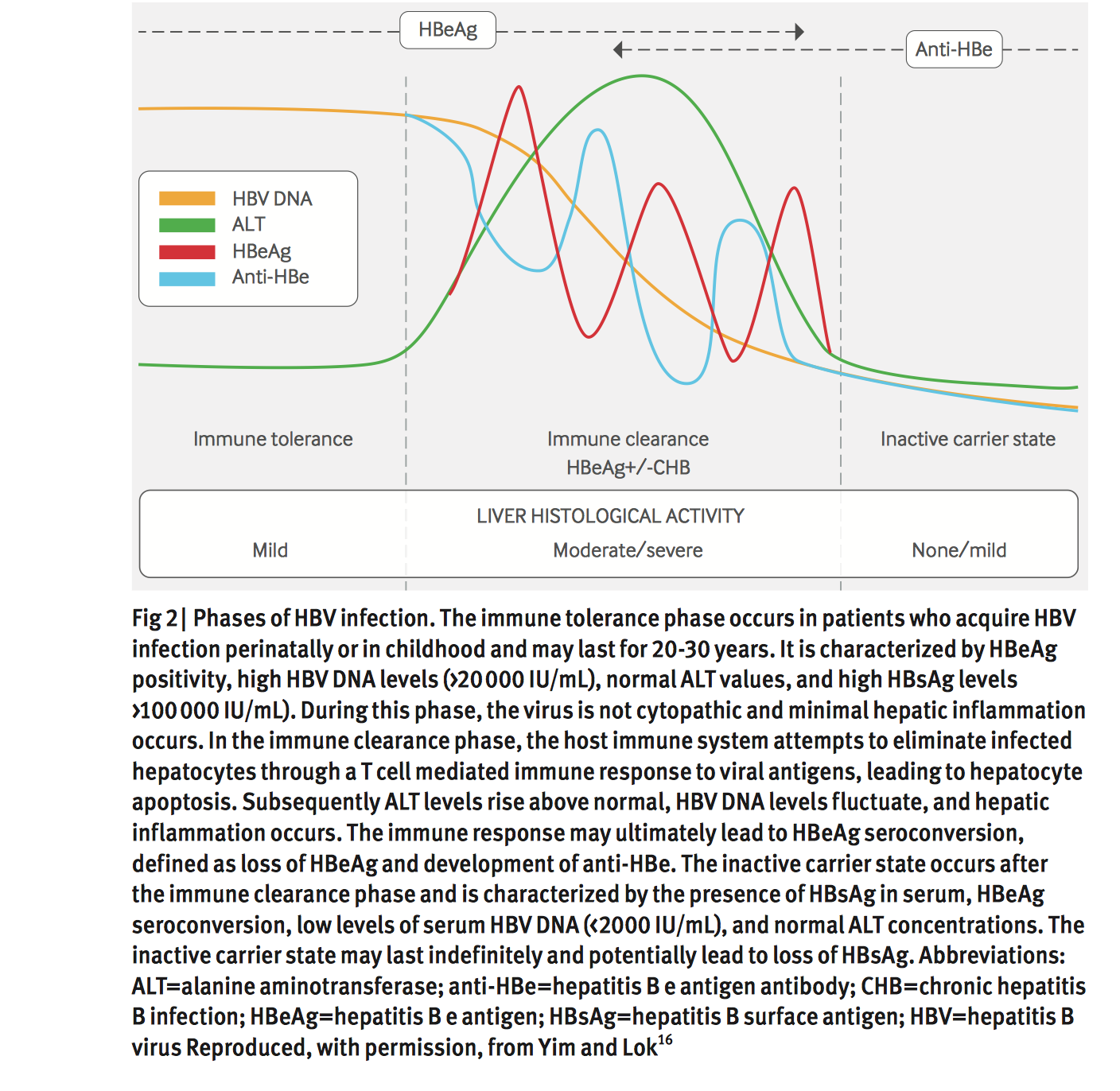
The National Institutes of Health recommends nucleoside therapy for the treatment of patients with acute liver failure, as well as cirrhotic patients who are HBV DNA positive and those with clinical complications, cirrhosis or advanced fibrosis with positive serum HBV DNA, or reactivation of chronic HBV during or after chemotherapy or immunosuppression. In addition, immunoglobulin and vaccination should be administered to newborns born to women positive for hepatitis B surface antigen .
In general, for hepatitis B e antigen -positive patients with evidence of chronic HBV disease, treatment is advised when the HBV DNA level is at or above 20,000 IU/mL and when serum ALT is elevated for 3-6 months.
For HBeAg-negative patients with chronic hepatitis B disease, treatment can be administered when the HBV DNA is at or above 2,000 IU/mL and the serum ALT is elevated for 3-6 months.
In patients coinfected with HBV and HIV, initiate therapy against HBV and administer antiretroviral therapy as well.
The NIH also indicates that immediate therapy is not routinely indicated for patients who have the following :
-
Chronic hepatitis B with high levels of serum HBV DNA but normal serum ALT levels or little activity on liver biopsy
-
Low levels of or no detectable serum HBV DNA and normal serum ALT levels
-
Positive serum HBV DNA but not HBsAg , unless the patient is undergoing immunosuppression
Read Also: Hepatitis B Is More Infectious Than Hiv True Or False
Nursing Support In The Management Of Chronic Viral Hepatitis
Specialist hepatitis nursing support is essential in the management of viral hepatitis. It is not an exaggeration to say that without nursing support, treatment of viral hepatitis, particularly hepatitis C, is not possible. Very few physicians have the time needed to educate patients and monitor them during treatment. This task has been assumed by specialist hepatitis and hepatology nurses who are now the main providers of care for and education of patients about their disease and about the treatment. The nurses teach self-injection and monitor patients on therapy. However, they also do much more. They provide support to the patient that busy physicians cannot, and they are more accessible than physicians. It is fair to say that by assuming many of the patient care responsibilities, they support both the patient and the physician. Currently, most hepatitis nurses in Canada are funded by the pharmaceutical industry. This results in a conflict of interest on the part of the nurse because his or her income comes from the company that makes the drug used. That only a minority of nursing positions across the country are publicly funded is unacceptable to doctors and nurses, and should be unacceptable to government.
Recommendation 4: Publicly funded comprehensive hepatitis nursing programs should be instituted in all provinces as a matter of urgency .
How Is Hepatitis C Treated
Hepatitis C is treated using antiviral drugs.
Treatment in the first 6 months focuses on:
- treating symptoms
- preventing the spread of the disease
- preventing complications, such as liver damage
For someone who has the disease beyond 6 months, treatment includes a combination of medications. However, not everyone with this form of hepatitis C will need treatment.
Whether or not you are getting treatment, you can help lower the risk of damage to your liver by:
- avoiding alcohol
Don’t Miss: What Are The Symptoms Of Hepatitis C Infection
Selection Of Patients For Treatment
In summary, the decision to treat requires the consideration of several factors: patient age, the level of viral replication, HBeAg status, and evidence of significant liver disease in the form of prolonged elevation of ALT level, fibrosis or inflammation on biopsy, or ultrasound evidence of cirrhosis.
Young adults who are HBeAg-positive usually have very high viral loads , with variable ALT levels . Most often, these individuals have no or minimal liver disease on biopsy. Immediate treatment may not be necessary, even with an elevated ALT level. It is impossible to predict whether these individuals will undergo seroconversion with remission of disease before the development of significant liver injury. In these patients, high viral loads do not carry the same implications for outcome as in older patients. Treatment can be withheld in the hope of seroconversion. However, these patients must be closely monitored because they may develop more severe disease. Thus, not every HBeAg-positive patient with an elevated ALT level needs treatment. provides an algorithm for identifying individual patients who may need treatment.
Recommendation 15: HBeAg-positive patients in whom the HBV DNA concentration is higher than 20,000 IU/mL with an elevated ALT level should be considered for treatment. Patients with significant inflammation or fibrosis on biopsy should also be treated, even if the HBV DNA concentration is lower than 20,000 IU/mL or the ALT level is normal .
Clinical Guidelines For Adults
The standard recommendation for care is to schedule visits with a liver specialist every six months, but this can be more or less depending on your medical situation. During these check-ups, the following usually occurs to monitor your health and your liver:
- Physical exam
On July 27, 2020, the American Society of Clinical Oncology published provisional guidelines recommending that all people diagnosed with cancer be tested for hepatitis B before starting anticancer treatment. According to the ASCO statement, up to 90% of people diagnosed with cancer have at least one risk factor for hepatitis B. Cancer treatments can suppress the immune system and cause the virus to reactivate, which can lead to serious liver damage or liver failure. The guidelines discuss how to manage people undergoing cancer treatment who also have hepatitis B, to prevent viral flares.
Recommended Reading: Can You Donate Blood If You Had Hepatitis A
Clinical Course Of Hcv Infection In Pediatric Patients
The transmission of HCV is through blood contact. Before widespread blood screening efforts were established in 1992, transmission of HCV to pediatric patients occurred mostly through blood transfusions and organ transplantations. After 1992, vertical transmission has become the most common means of HCV transmission in children. Approximately 7,500 new cases of CHC occur annually in the US from vertical transmission. There is increasing concern for horizontal transmission through injection drug use, especially among adolescents. HCV infection rates are rising among adolescents and young adults in the US, especially in eastern rural regions of the country. This is associated with the opioid epidemic and increased use of injected opiates. As these individuals are entering childbearing age, the risk of vertical transmission will also increase. Spontaneous clearance of infection occurs in 25%40% of infected infants but occurs far less frequently in older children . Thus, the majority of children exposed to HCV will progress to develop CHC.
Hepatitis B: Screening Prevention Diagnosis And Treatment
THAD WILKINS, MD, MBA RICHARD SAMS, MD, MA and MARY CARPENTER, PharmD Medical College of Georgia at Augusta University, Augusta, Georgia
Am Fam Physician. 2019 Mar 1 99:314-323.
Patient information: See related handout on hepatitis B, written by the authors of this article.
The Centers for Disease Control and Prevention estimated that in 2015 there were 21,900 cases of acute hepatitis B, with an overall incidence of 1.1 cases per 100,000.1 There are an estimated 850,000 to 2.2 million individuals in the United States with chronic hepatitis B.1,2 Approximately 25% of children and 15% of adults with chronic hepatitis B die prematurely from hepatocellular carcinoma or cirrhosis.3 However, treatment reduces morbidity and mortality from the disease.
WHAT IS NEW ON THIS TOPIC
Approximately 1,000 cases of perinatal hepatitis B occur annually in the United States, and nearly 90% of chronic hepatitis B cases in infants develop in the first year of life.
Hepatitis B vaccination is recommended for all medically stable infants weighing 2,000 g or more within 24 hours of birth, unvaccinated infants and children, and unvaccinated adults requesting protection from hepatitis B or who are at increased risk of hepatitis B.
SORT: KEY RECOMMENDATIONS FOR PRACTICE
Pregnant women should be screened for hepatitis B at the first prenatal visit.
SORT: KEY RECOMMENDATIONS FOR PRACTICE
Pregnant women should be screened for hepatitis B at the first prenatal visit.
eFIGURE A
eFIGURE A
Also Check: How Can You Treat Hepatitis C
How Can I Avoid Getting Hepatitis B
There is a safe and effective vaccine that can protect you from getting hepatitis B. The vaccine is usually given in three doses over a six month period. The vaccine will give you long-lasting protection. A combined vaccine for hepatitis A and hepatitis B is also available.
Other ways to protect yourself or your loved ones include:
- Adopt safe sex practices.
- Avoid sharing personal hygiene items
- If you have been exposed to the hepatitis B virus , an injection of hepatitis B immune globulin may help protect you.
- If you are pregnant, make sure you are screened for hepatitis B. If the test result shows that you have the virus, make sure your baby receives the free hepatitis B vaccine. If you have hepatitis B, breastfeeding is safe if the baby has received both protective antibody called immune globulin, and the first dose of hepatitis B vaccine within the first 12 hours of life. Talk to your doctor about having your newborn immunized .
- If you decide to have a tattoo, piercing, manicure or pedicure, ensure that the facility uses single-use needles and inks and/or follows proper sterilization procedures.
Antiviral Medication For Hepatitis C

For people with hepatitis C, the goal of treatment with antiviral medication is to prevent the virus from replicating, or copying itself, and to eliminate the virus from the bloodstream. If the hepatitis C virus has been in the body for more than six months, the infection is considered chronic. Without treatment, most people with acute hepatitis C develop the chronic form of the disease.
Your doctor decides which antiviral medicationor combination of medicationsto prescribe based on the results of a blood test called a genotype test. There are six genotypes, or strains, of the hepatitis C virus, and people with certain genotypes respond more quickly to medical treatment.
For many years, the standard treatment for chronic hepatitis C consisted of the antiviral medications pegylated interferon and ribavirin. Ribavirin is taken by mouth every day, and interferon is an injection that you or a caregiver can administer once a week at home.
In 2013 and 2014, the U.S. Food and Drug Administration approved a group of new medications for the treatment of hepatitis C. These medications, which include sofosbuvir, are very effective and have fewer side effects than older medications, particularly interferon.
Recommended Reading: What Does Immunity To Hepatitis B Mean
Ldv/sof Use In Adults
The introduction of DAAs in 2011 revolutionized CHC treatment in adults, and nowadays CHC in adults is considered curable. There are currently ten different oral regimens licensed by the EMA and FDA for treatment of CHC in adults. All regimens achieve high sustained virologic response rates with 12 weeks of therapy, regardless of genotype, stage of fibrosis, or presence of human immunodeficiency virus infection. SVR is defined as undetectable HCV RNA after completion of therapy, usually evaluated 12 weeks after treatment completion and coined SVR12. Certain populations can even achieve equally high SVR rates with only 8 weeks of therapy.
LDV/SOF has shown great safety and in treatment-naïve and treatment-experienced adult patients with CHC with all genotypes. Pooled analysis of the ION trials found that treatment-emergent adverse events occurred in 74% of 1,080 LDV/SOF patients, and 85% of LDV/SOF with ribavirin patients, with most TEAEs mild to moderate in severity. Very few of patients discontinued treatment due to TEAEs. The most common treatment-related adverse events were fatigue , headache , nausea , diarrhea , and insomnia , most of which were grade 1 in severity. Treatment-emergent serious adverse events occurred in 2% of LDV/SOF patients and 3% of LDV/SOF with ribavirin patients of these, 0.4% were considered treatment-related.
Antiviral Medication For Hepatitis B
Doctors may recommend antiviral medication for people with chronic hepatitis B, which occurs when the virus stays in your body for more than six months.
Antiviral medication prevents the virus from replicating, or creating copies of itself, and may prevent progressive liver damage. Currently available medications can treat hepatitis B with a low risk of serious side effects.
NYU Langone hepatologists and infectious disease specialists prescribe medication when they have determined that without treatment, the hepatitis B virus is very likely to damage the liver over time. People with chronic hepatitis B may need to take antiviral medication for the rest of their lives to prevent liver damage.
There are many different types of antiviral medications available, and your doctor recommends the right type for you based on your symptoms, your overall health, and the results of diagnostic tests. A doctor may take a wait-and-see approach with a person who has a healthy liver and whose blood tests indicate a low viral load, the number of copies of the hepatitis B virus in your bloodstream.
Someone with HIV infection or AIDS may have a weakened immune system and is therefore more likely to develop liver damage. The U.S. Centers for Disease Control and Prevention strongly recommends that people with HIV infection who are diagnosed with hepatitis B immediately begin treatment with antiviral medication.
Read Also: How Do You Get Hepatitis Ab And C