Recovery Of Metabolic Damage
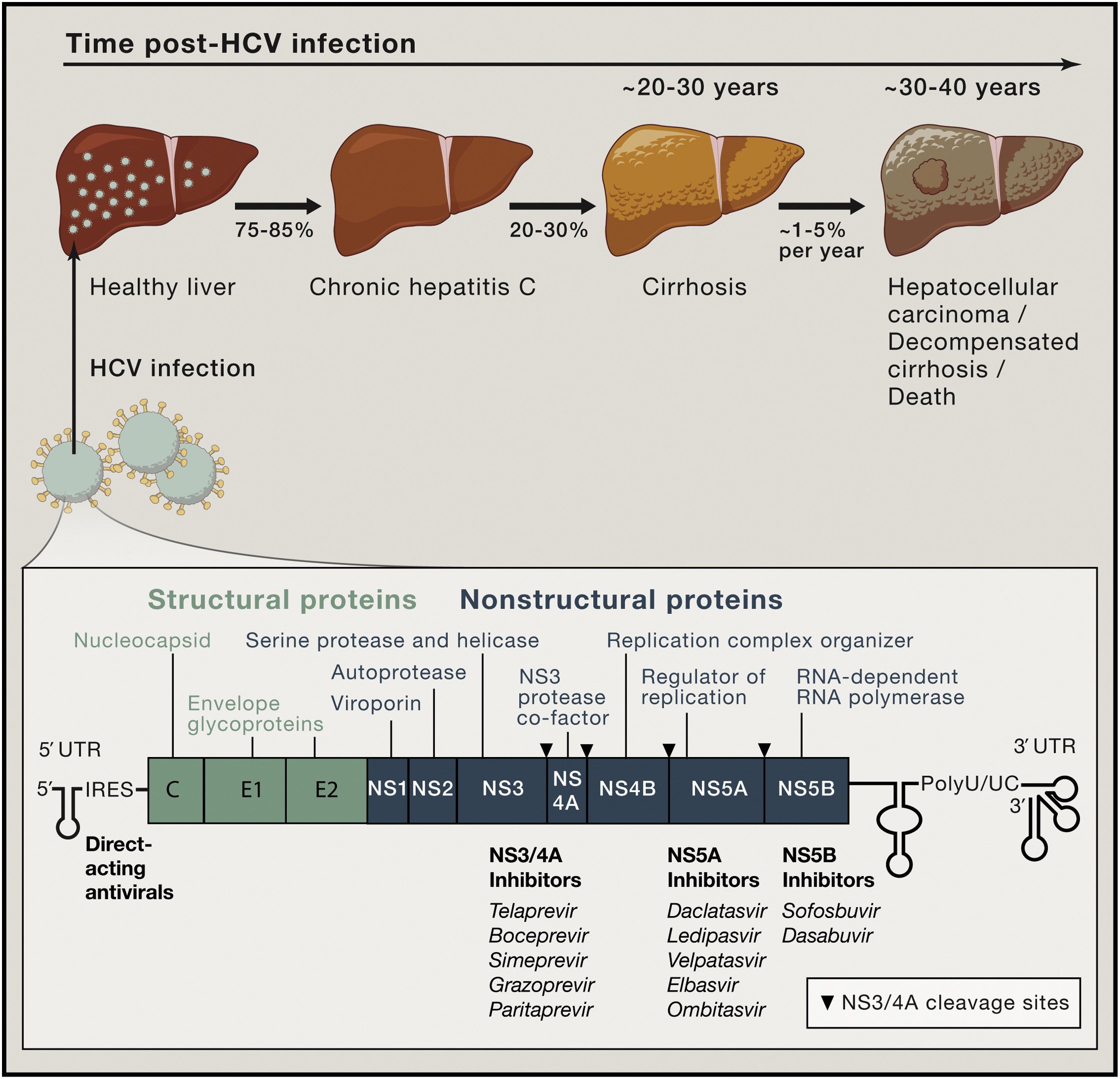
The association between HCV infection and dysregulation of metabolic processes has been observed since long ago. Furthermore, chronic HCV infection exerts a significant impact on the development of heart disease and stroke . Increasing epidemiological studies have long demonstrated that the prevalence of type 2 diabetes mellitus is much higher in subjects with chronic hepatitis C than in the general population, ranging between 13% and 67% according to liver fibrosis stage and time of infection .
The hypothesis that HCV has a direct and important role in the regulation of glucose metabolism is supported by laboratorial investigations. Kasai D et al. showed that HCV replication can down- regulate the glucose transporter 2 expressions, which located on the surface of the cell, thereby affecting cellular uptake of glucose . Deng L et al. found that HCV up-regulated hepatic glucose production via NS5A-mediated FoxO1-dependent pathway . Recently, more systematic mechanisms underlying disorders of glucose metabolism caused by HCV infection have been observed in many experimental and clinical studies. HCV may directly inhibit the insulin-signaling pathway, with downregulation of glucose transporter 2, promotion of IRS-1 degradation through protein kinase B /mammalian target of rapamycin activation, and suppression of phosphorylation of tyrosine on IRS-1. Moreover, HCV impairs phosphorylation of Akt, leading to a reduction in insulin stimulation .
Figure 1
Metabolic Measures And Hcv Treatmentall Patients
The means of the metabolic measures across the study period are presented in Table 2 and changes from baseline at each time point are reported in Figure 1, Figure 2, Figure 3 and Figure 4. Changes were observed for HbA1c and lipid measures across the study period . Overall, glucose , insulin , and HOMA-IR did not change over time . Individual HOMA-IR trajectories over time are presented in Figure 1.
Homeostatic Model AssessmentInsulin Resistance at each study time point for each participant by treatment group treatment in participants without cirrhosis, RBV = ribavirin free HCV treatment in participants without cirrhosis, RBV+F4+ = ribavirin containing HCV treatment in participants with cirrhosis).
Pharmacologic Basis Of Direct Acting Antiviral Drug Interaction
Drug-drug interaction is the modification of the action of one drug by another and may be pharmacodynamic or pharmacokinetic in nature. Pharmacodynamic interactions dont result due to a change in drug concentrations but lead to alteration of the response of body to another drug. Pharmacodynamic interactions may occur between drugs with opposite actions leading to antagonism or may occur between drugs with similar actions leading to potentiation, addition or synergism. Pharmacodynamic interactions are not easy to be quantified, and subsequently, are difficult to manage. For example, during the interferon-based therapy for chronic HCV, Peg-INF enhances the ribavirin-induced anemia to discontinue the therapy. On the other hand, pharmacokinetic interactions do result in a change in drug concentrations and occur at the level of drug absorption, distribution, metabolism and elimination. Pharmacokinetic interactions are partially predictable based on the available data and can be limited or prevented by a dosage adjustment.
Recommended Reading: How Is Hepatitis D Transmitted
Impact Of Successful Treatment With Direct
-
* E-mail:
Affiliations Department of Pharmacy, Complejo Hospitalario de Navarra, Pamplona, Spain, Instituto de Salud Pública de NavarraIdiSNA, Pamplona, Spain, CIBER Epidemiología y Salud Pública , Pamplona, Spain
- Iván Martínez-Baz,
Roles Formal analysis, Methodology, Writing review & editing
Affiliations Instituto de Salud Pública de NavarraIdiSNA, Pamplona, Spain, CIBER Epidemiología y Salud Pública , Pamplona, Spain
-
Roles Conceptualization, Methodology, Supervision, Writing review & editing
Affiliation Department of Pharmacy, Complejo Hospitalario de Navarra, Pamplona, Spain
-
Roles Methodology, Resources, Writing review & editing
Affiliation Department of Gastroenterology, Complejo Hospitalario de Navarra, Pamplona, Spain
-
Roles Methodology, Resources, Writing review & editing
Affiliation Department of Economics, Public University of Navarra, Pamplona, Spain
- Jesús Castilla
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing review & editing
Affiliations Instituto de Salud Pública de NavarraIdiSNA, Pamplona, Spain, CIBER Epidemiología y Salud Pública , Pamplona, Spain
Side Effects Of Treatment
Treatments with direct-acting antivirals have very few side effects. Most people find DAA tablets very easy to take.
You may feel a little sick and have trouble sleeping to begin with, but this should soon settle down.
Your nurse or doctor should be able to suggest things to help ease any discomfort.
You need to complete the full course of treatment to ensure you clear the hepatitis C virus from your body.
If you have any problems with your medicines, speak to your doctor or nurse straight away.
Side effects for each type of treatment can vary from person to person.
For a very small number of people, more severe side effects from hepatitis C treatments may include:
Recommended Reading: How Can Hepatitis C Be Transmitted
Data Collection And Analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were hepatitis Crelated morbidity, serious adverse events, and quality of life. Our secondary outcomes were allcause mortality, ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, hepatocellular carcinoma, nonserious adverse events , and sustained virological response. We systematically assessed risks of bias, performed Trial Sequential Analysis, and followed an eightstep procedure to assess thresholds for statistical and clinical significance. The overall quality of the evidence was evaluated using GRADE.
Overview Of Direct Acting Antiviral Drug Interactions
The introduction of direct acting antiviral agents for treatment of hepatitis C viral infections has achieved high virological cure rates encouraging more patients to start thetreatment. However, the wide use of combined DAAs regimens offers a possibility of exposure to drug interactions especially in patients under treatment for other co-morbidities. It is not only important to monitor for drug-drug interaction during HCV therapy with DAAs, but also to evaluate the interaction potential even before the start of treatment course in order to limit the drug interaction. Indeed, this is a complex issue given the large number of drug classes that may be prescribed or non-prescribed and the combined DAAs regimens particularly those including protease inhibitors. Moreover, the disease stage of liver that itself is the target for DAAs therapy and is the common site of drug-drug interactions. Direct acting antiviral could be a victim, as they are substrates and/or a culprit and they may act as enzyme inhibitors or inducers in drug-drug interactions. Because DAAs are recently approved, most of the available data comes from in vitro research and there is a limitation in available clinical data regarding these drugs. With regard to this complex issue, there are online websites that provide help tools for the clinicians in exploring potential drug-drug interactions .
Don’t Miss: How Many People Have Hepatitis C
Access To Hepatitis C Care In Prisons
Direct-acting antivirals were listed on Australias Pharmaceutical Benefits Schedule in 2016. These subsidised medicines were made available to all Australians, including people in prison. Prisoners are usually excluded from the federal governments PBS subsidies, with medication costs falling to states and territories.
While overall hepatitis C treatment rates stagnated in Australia, the prison sector accounted for a rising percentage of all people treated. Between March 2016 and February 2017, around 6% of all hepatitis C treatments occurred in Australian prisons. In 2020, this rose to 37% .
For some people, prison is one of few places they can receive hepatitis C treatment.
A pilot evaluation of a nurse outreach program in Victorian prisons found of the 416 people who started direct-acting antiviral treatment, most had never had hepatitis C care before.
An additional 75 people were released from prison before they could start treatment. After referral to their preferred physician, only 19 were prescribed direct-acting antivirals within six months of release. Seven of those people were treated only after they were re-incarcerated.
Many people leaving prison face multiple challenges, including housing instability, poverty, obtaining meaningful and reliable employment, and social connectedness. These are all potential barriers to accessing health care, including hepatitis C treatment.
Safety And Efficacy Of Direct
1Department of Tropical Medicine and Gastroenterology, Faculty of Medicine, South Valley University, Qena, Egypt. 2Department of Clinical Pathology, Faculty of Medicine, South Valley University, Qena, Egypt 3Department of Internal Medicine, Faculty of Medicine, Sohag University, Sohag, Egypt Abstract Keywords
Recommended Reading: Hepatitis B Labs To Order
Challenges And Future Direction Of Daa
Though DAA has provided much needed, safe and effective therapeutic option for chronic HCV patient, some challenges need further effort. Such challenges include the presence of resistant variance, low efficacy in cirrhotic patients, presence of drug-drug interactions, and the cost. The future direction should go through multiple directions, for example, continuous monitoring and developing DAA, use of combined groups of DAA with different mechanisms of action to minimize resistance, searching for other antiviral groups with different mechanisms of action, and finding a solution for improving cirrhosis by developing antifibrotic drugs. A recent study showed promising results in the possible incorporation of a new cyclophilin inhibitor, STG-175 in DAA-regimen.
Ethics Approval And Consent To Participate
All procedures performed in this study were in accordance with the ethical standards of Ain Shams University Research Committee and with the 1964 Helsinki declaration and its later amendments.
Ethics committees reference number: 000017585.
Address: Faculty of Medicine, Ain Shams University, Cairo 11211, Egypt.
Informed written consent was obtained from each participant before enrolment in the study.
Also Check: Herbal Medicine For Hepatitis B In The Philippines
Ethics Approval And Informed Consent
The present study was conducted in accordance with the Declaration of Helsinki. Permission obtained from Research Ethics Committee, faculty of medicine, Tanta University, Egypt . An informed written consent was obtained from all participants in this research. Privacy of all patients data was granted by a special code number for every patients file that includes all investigations. The results of the research were used only in scientific purposes and not in any other aims.
Evidence For Hypoglycaemia During Direct
Studies show that some patients with diabetes initiating direct-acting antiviral therapy for hepatitis C have experienced hypoglycaemia. The studies indicate that achieving sustained virological response is associated with improvements in glycaemic control, compared to patients who relapse or are non-responders. Many studies recorded these changes in glycaemic control in the first 3 months of treatment. Some studies reported the need to adjust patientâs diabetic medication following changes in glucose metabolism, with up to 30% of patients requiring adjustments to their treatment.
An EU review confirmed the risk of hypoglycaemia in patients with diabetes who had been initiated on direct-acting antivirals for chronic hepatitis C. Information on the risk is being added to the Summary of Product Characteristics and Patient Information Leaflet for these medicines.
Patients with diabetes should be closely monitored for changes in glucose levels, particularly in the first 3 months of treatment, and adjustments to their diabetic medication or doses made where necessary.
Recommended Reading: Best Food For Hepatitis C
Cirrhosis And Metabolic Measures
Participants with cirrhosis had higher baseline insulin and HOMA-IR than non-cirrhotic patients in the RBV-sparing group . These measures remained unchanged from baseline while on treatment and 12 weeks following dosing in the overall study population as well as in cirrhotic and non-cirrhotic participants. Twenty-four weeks post-treatment, an increase in insulin and HOMA-IR was noted in cirrhotic participants, which was not observed in non-cirrhotic participants. When the results of a single outlier were removed, this finding was no longer present . Participants with cirrhosis had similar trajectories of HbA1c and lipid measures compared to those in the non-cirrhotic ribavirin-containing treatment group .
apoA1, apoA2, and apoE were lower at baseline in the cirrhotic participants compared with non-cirrhotic participants . There were no observed changes from baseline in mean glucose and apolipoprotein measures among cirrhotic patients over the study period.
Update On Hepatitis C: Direct
Correspondence to: Aijaz Ahmed, MD, Associate Professor of Medicine, Medical Director Liver Transplant Program, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, 750 Welch Road, Suite 210, Stanford, CA 94305, United States.
Telephone: +1-650-4986091 Fax: +1-650-4985692
Recommended Reading: Hepato Liver Support For Dogs
Pregnancy And Hepatitis C
The new hepatitis C medicines have not been tested in pregnancy.
You should not become pregnant while taking treatment as it could be harmful to unborn babies.
If you’re pregnant, you must delay treatment until after your baby is born.
Speak to your doctor before starting hepatitis C treatment if you’re planning to become pregnant in the near future.
You’ll need to wait several weeks after treatment has ended before trying to get pregnant.
Women taking ribavirin should use contraception during treatment and for another 4 months after the end of treatment.
If you become pregnant during treatment, speak to your doctor as soon as possible to discuss your treatment options.
Injecting Drug Use In Prisons
Imprisonment enables some people to stop using drugs, but others continue to inject, and some start injecting.
No Australian jurisdiction provides sterile injecting equipment to people in prison, despite this being available in the community. The likelihood of syringe sharing in prisons is therefore high, and increases the risk of hepatitis C transmission.
One NSW study estimated 10% of people who injected drugs in prison were newly infected each year.
Another study found recent incarceration increases the risk of contracting hepatitis C by 62%.
Recommended Reading: What Does The Hepatitis B Vaccine Prevent
Clinical Pharmacology Of Newer Daa
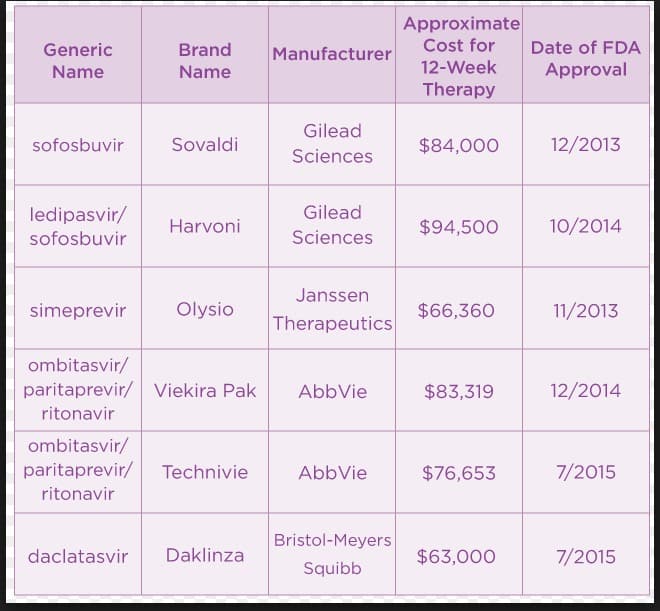
The direct acting antiviral therapy was started when the first-wave, first-generation HCV NS3-4A protease inhibitors boceprevir and telaprevir were approved in combination with PegIFN-alpha and ribavirin for the treatment of chronic HCV genotype 1 infection in 2011.However, these drugs have been reported to have several drug-drug interactions, and are largely replaced with newer DAA including Simeprevir, Paritaprevir, Daclatasvir, Ledipasvir, Ombitasvir, Sofosbuvir and Dasabuvir. Table 3 summarizes the pharmacokinetics of all approved DAA with special reference to the sites of drug-drug interaction. Clinical pharmacology of the newer DAA will be discussed in the next section.
High Rates Of Drug Use Among Those Entering Prison
In Australia and many other countries, the criminalisation of drug use results in the frequent incarceration of people who inject drugs. About half of people entering prison report a history of injecting drugs.
While drug courts and diversion programs help keep some people out of prison, more needs to be done to treat drug use as a health issue rather than a criminal one.
The over-incarceration of people who inject drugs results in high rates of hepatitis C among the prison population. In 2016, of people entering prison who reported injecting drugs, approximately 50% had been exposed to hepatitis C but not all may have had an active infection. This compares with less than 1% of those entering prison who did not report injecting.
Read Also: What Is Hepatitis C Virus Ab
Advice For Healthcare Professionals:
- rapid reduction in hepatitis C viral load during direct-acting antiviral therapy for hepatitis C may lead to improvements in glucose metabolism in patients with diabetes, potentially resulting in symptomatic hypoglycaemia if diabetic treatment is continued at the same dose
- be vigilant for changes in glucose tolerance and advise patients of the risk of hypoglycaemia during direct-acting antiviral therapy, particularly within the first 3 months when the viral load is being reduced, and modify diabetic medication or doses when necessary
- physicians who initiate direct-acting antiviral therapy in patients with diabetes should inform the healthcare professional in charge of the diabetic care of the patient
- report any suspected adverse drug reactions associated with direct-acting antiviral therapies to the Yellow Card Scheme without delay
Statistical Analysis Of Data
The data was collected, presented and statistically analyzed with the computer program SPSS version 19 . For quantitative data, the Kolmogorov test for normality was performed. For normally distributed data, values were expressed as mean and independent samples t test was performed for comparison between two groups. For data that were not normally distributed median and interquartile range were calculated and MannWhitney test , and Spearmans rank-order correlation were used. For qualitative data, Pearsons Chi square test was used to examine association between two variables. Significance was adopted at probability value < 0.05 for interpretation of results of tests.18
You May Like: Hepatitis C Transmission Through Kissing
Ribavirin And Metabolic Measures
Ribavirin-containing treatment was associated with a decrease in LDL-C at Weeks 4 and 12 . This effect did not persist post-treatment. HbA1c decreased during treatment in the ribavirin-exposed group , but this change was not sustained post-treatment. No differences in HOMA-IR at each time point were observed in ribavirin-exposed participants compared to those who did not receive ribavirin. Compared to baseline, on-treatment decreases were observed at Week 4 in apoA2 and apoB or at Weeks 4 and 12 in apoE in ribavirin recipients .
The Interplay Between Direct
Sun Hong Yoo
Department of Internal Medicine, Catholic University Liver Research Center, The Catholic University of Korea , Seoul 06591, Korea .
Correspondence Address: Prof. Jung Hyun Kwon, Department of Internal Medicine, Catholic University Liver Research Center, The Catholic University of Korea, Seoul, Korea. E-mail: doctorkwon@catholic.ac.kr
Received:First Decision:Revised:Accepted:Science Editor:Copy Editor:Production Editor:
© The Author 2020. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author and the source, provide a link to the Creative Commons license, and indicate if changes were made.
You May Like: What Is The Worst Hepatitis
Rehabilitation Of Immune Damage
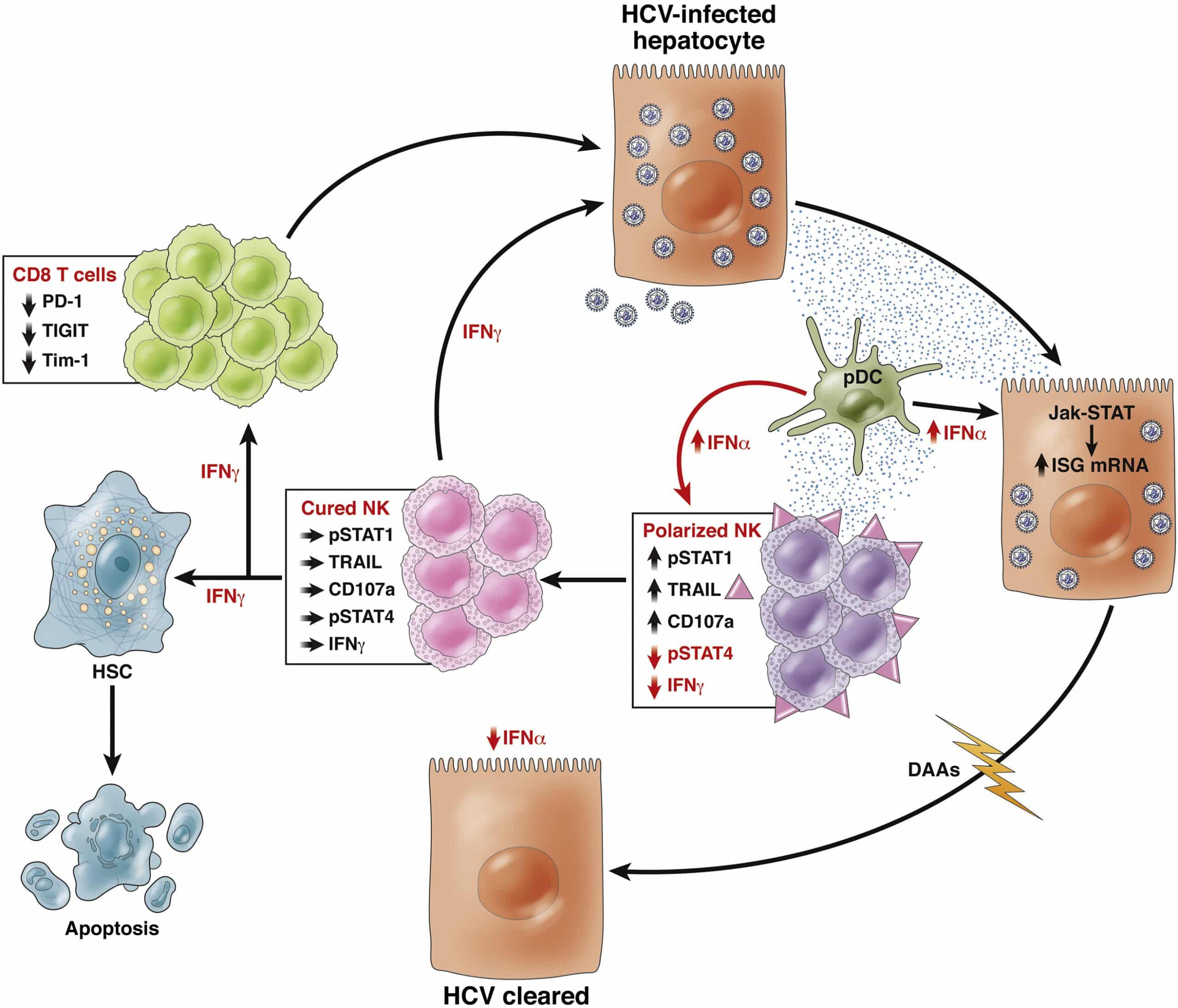
A great deal of studies have shown that HCV infection will induce the up-regulation of many genes involved in innate immunity characterized by up-regulation of interferon-stimulated genes expression , elevated levels of interferon- sensitive cytokines and chemokines . More importantly, the chronic activation of the innate immune response and the consequent activation of hepatic stellate cells are the initiators of hepatitis and cirrhosis . HCV may interact with immunity response through multiple mechanisms. It is well known that HCV RNA can be recognized by the Toll-like receptor 3 or the RIG-I helicase-mediated pathway in the cytoplasm, resulting in transcriptional activation of type 1 interferon. And type 1 interferon can activate the JAK-STAT signal pathway, which subsequently precedes the transcription of ISGs that have antiviral effects . Simultaneously, the increased type 1 IFN can also trigger natural killer cells and make it a polarized phenotype, elevated cytotoxicity, and down-regulation of the pro- apoptotic factors TRAIL and cytokines .
Unlike interferon-based treatments, DAA treatment acts precisely on some critical steps of HCV replication, thereby preventing HCV replication. It plays a role in the treatment of CHC less dependent on the host’s immune function. Hence, many researchers speculate that the dysfunction of CHC patient’s immune system may be recovered partially or completely with the DAA clearing HCV.
Figure 2