Hiv And Hbv Coinfection
About 2% of people with HIV in the United States are coinfected with HBV both infections have similar routes of transmission. People with HIV are at greater risk for complications and death from HBV infection. All people with HIV are recommended to be tested for HBV, and if susceptible, are further recommended to receive the hepatitis B vaccination or, if chronically infected, evaluated for treatment to prevent liver disease and liver cancer. For more information about HIV and HBV coinfection, visit HIV.govâs pages about hepatitis B and HIV coinfection.
Persons New To Canada
Health care providers who see persons newly arrived in Canada should review the immunization status and update immunization for these individuals, as necessary. In many countries outside of Canada, HB vaccine is in limited use.
All persons from a country that is endemic for HB should be assessed and vaccinated against HB if not immune and not infected. Individuals born in developing countries are more likely to be carriers of HB, necessitating vaccination of their sexual and household contacts based on review of their serologic test results. HB vaccine is recommended for all household contacts whose families have immigrated to Canada from areas in which there is a high prevalence of HB and who may be exposed to HB carriers through their extended families or when visiting their country of origin.
Children adopted from countries in which there is a high prevalence of HB infection should be screened for HBsAg and, if positive, household or close contacts in the adopting family should be immunized before adoption or as soon as possible thereafter. Adults going to pick-up children from these countries should be vaccinated before departure. Refer to Immunization of Persons New to Canada in Part 3 for additional information.
Who Should Get Hepatitis Vaccinations
Since the vaccines were first developed, the hepatitis A and B vaccines have become part of the regular childhood immunization schedule. They are not considered a routine adult immunization.
“When we’re talking about adults, I would say yes, get the vaccine if they fit into one of these risk factors” says Poland. “If they don’t fit into the risk factors, their risk is so low that there’s no compelling reason to do it.”
People at risk for hepatitis A include:
- Anyone traveling to or working in areas where hepatitis A is more widespread.
- People whose work puts them in potential contact with hepatitis A, such as those who work with the hepatitis A virus in research labs
- People who are treated with clotting-factor concentrates
- People who have chronic liver disease
- People who use recreational drugs, injected or not
- Men who have sex with men
People at risk for hepatitis B include:
- Anyone traveling to or working in areas where hepatitis B is more widespread.
- Health care workers and other people whose job exposes them to human blood
- People with HIV infection, end-stage kidney disease, or chronic liver disease
- People who live with someone with hepatitis B
- People who inject street drugs
- Sexually active people who have had more than one partner
- Anyone who has had an STD
- Men who have sex with men
- Sex partners of people with hepatitis B
You May Like: Hepatitis B Antibody Test Cost
Accelerated Us Children And Adult Hepatitis B Vaccine Schedules
*Please note that the first dose should be given as soon as possible. Additional doses require minimum time intervals between doses in order for the vaccine to be effective.
In some instances, it may be necessary to vaccinate within a short period of time to ensure protection before travel. There are accelerated schedules to provide the highest level of protection over a short period of time. Individuals who need an accelerated schedule must have a booster dose at 1 year to ensure long-term protection. Note that the 2-dose Heplisav-B vaccine will also ensure maximum protection over a 1-month period without the need for a booster dose at 1 year.
4-Dose Vaccine Series for Children and Adults
Engerix-B is a 3-dose vaccine that can be given on an accelerated, four-dose schedule, with 3 shots administered within 2 months, and a booster dose at 1 year to provide maximum long-term protection.
4-Dose Combination Hepatitis A and B Vaccine Series
Twinrix is a 4-dose vaccine that can be given on an accelerated schedule to provide protection against hepatitis A and B. Three doses are administered within 1 month, followed by a booster shot at 1 year. This is a common choice of vaccine for those travelling on short-notice outside the U.S. It is important to complete the booster dose at 1 year, to ensure long-term protection.
2-Dose Vaccine Series
Serological Testing After Hepatitis B Vaccination

It is recommended that levels of hepatitis B surface antigen in infants born to mothers with chronic hepatitis B are measured 312 months after they complete the primary vaccine course. Do not test the infant before 9 months of age, to avoid detecting anti-HBs
Post-vaccination serological testing is recommended 48 weeks after completing the primary course for:
- people at significant occupational risk, such as healthcare workers whose work involves frequent exposure to human tissue, blood or body fluids
- people at risk of severe or complicated hepatitis B, such as people who are immunocompromised and people with pre-existing liver disease not related to hepatitis B
- people who may respond poorly to hepatitis B vaccination, such as haemodialysis patients and people with bleeding disorders who received the vaccine subcutaneously
- close contacts of people who are infected with hepatitis B virus, including sexual partners, household contacts and household-like contacts22
If serological testing 48 weeks after the primary course shows levels of antibody to hepatitis B surface antigen of < 10 mIU per mL, check the person for acute or chronic hepatitis B virus infection by testing for serological markers, including antibodies to anti-HBs and hepatitis B core antigen.
After the booster dose, check for anti-HBs
A non-responder is a person who:
Read Also: How Does Hepatitis Affect The Body
International Hepatitis B Vaccine Schedules
*Please note that the first dose should be given as soon as possible. Additional doses require minimum time intervals between doses in order for the vaccine to be effective.
The hepatitis B vaccine is an injection that is generally given in the arm and as a three-dose series. The World Health Organization recommends a 0, 1, and 6-month vaccine schedule, though schedules may vary based on a countrys national immunization program. Completing the hepatitis B vaccine series, preferably beginning at birth, will ensure protection against hepatitis B, hepatitis delta and lower the lifetime risk of liver cancer. Greater than 90% of babies and up to 50% of young children who are not vaccinated and are infected with hepatitis B will have lifelong infection, which makes the birth dose essential to their protection. Please note that the vaccine brand name, manufacturer and associated schedules for adults, children and infants may be unique to different countries, though there is a list of WHO prequalified vaccines.
3-Dose Vaccine Series for Infants
The World Health Organization recommends all infants receive the first dose of the hepatitis B vaccine within 24 hours of birth and to complete the vaccine series with additional shots at 1 month and 6 months of age. Beginning the hepatitis B vaccine at birth will ensure protection against hepatitis B for life.
3-Dose Vaccine Series for Children and Adults
4-Dose Combination Vaccine Series for Infants
Additional Resource Links:
How To Get Vaccinated Against Hepatitis B
All babies in the UK born on or after 1 August 2017 are given 3 doses of hepatitis B-containing vaccine as part of the NHS routine vaccination schedule. These doses are given at 8, 12 and 16 weeks of age.
Babies at high risk of developing hepatitis B infection from infected mothers are given additional doses of the hepatitis B vaccine at birth, 4 weeks and 1 year of age.
If you think you’re at risk and need the hepatitis B vaccine, ask your GP to vaccinate you, or visit any sexual health or genitourinary medicine clinic.
If your GP or nurse is unable to offer you the hepatitis B vaccine because of a temporary shortage in supply, you may need to wait longer for the vaccine. For more information, read What to do if you have to wait for a dose of hepatitis B vaccine .
If your job places you at risk of hepatitis B infection, it’s your employer’s responsibility to arrange vaccination for you, rather than your GP. Contact your occupational health department.
Also Check: Can Patients With Impaired Hepatic Function Be Treated With Buprenorphine
Guidance On Reporting Adverse Events Following Immunization
Vaccine providers are asked to report, through local public health officials, any serious or unexpected adverse event temporally related to vaccination. An unexpected AEFI is an event that is not listed in available product information but may be due to the immunization, or a change in the frequency of a known AEFI.
Refer to Reporting Adverse Events Following Immunization in Canada and Adverse events following immunization in Part 2 for additional information about AEFI reporting.
Hepatitis B Vaccine Complications
Adverse reactions to the vaccine are few and usually mild:
- There may be some soreness and erythema around the site. These are the most common reactions.
- Fatigue, malaise and influenza-like symptoms are rare.
- Rare associations with a Guillain-Barré-type syndrome and also multiple sclerosis have been reported but a causal relationship has not been substantiated.
HBIG is well tolerated. Reactions and side-effects are rare.
You May Like: What Is The Blood Test For Hepatitis C
Adults Recommended To Receive Hepb Vaccine:
- Persons at risk for infection by sexual exposure
- Sex partners of hepatitis B surface antigen positive persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship
- Persons seeking evaluation or treatment for a sexually transmitted infection
- Men who have sex with men
Immunizing Agents Available For Use In Canada
Hepatitis A-containing vaccines
- AVAXIM® and AVAXIM®-Pediatric , Sanofi Pasteur SA , Sanofi Pasteur Ltd.
- HAVRIX®1440 and HAVRIX®720 Junior , GlaxoSmithKline Inc.
- TWINRIX® and TWINRIX®Junior , GlaxoSmithKline Inc. Refer to Hepatitis B Vaccine in Part 4 for additional information about HAHB vaccine.
- VAQTA® , Merck Canada Inc.
- ViVAXIM® , Sanofi Pasteur Ltd.
Human immunoglobulin
- GamaSTAN® , Grifols Therapeutics LLC.
Standard human immunoglobulin is a sterile, concentrated solution for intramuscular injection containing 15% to 18% immunoglobulin. It is obtained from pooled human plasma from screened donors and contains mainly IgG with small amounts of IgA and IgM. For complete prescribing information, consult the product leaflet or information contained within the product monograph available through Health Canada’s Drug product database.
Refer to Contents in Immunizing Agents Available for Use in Canada in Part 1 for lists of vaccines and passive immunizing agents available for use in Canada and their contents.
Recommended Reading: Hepatitis C Antibody With Reflex
Why Do You Need A Hepatitis B Shot
Hepatitis B is a viral infection that cant be transferred person-to-person unless you have contact with an infected persons bodily fluids. Annual infection rates of HBV are going down in the United States thanks to vaccines. So you might be wondering if you or your child needs a shot to protect against hepatitis B.
Who Is Most Affected
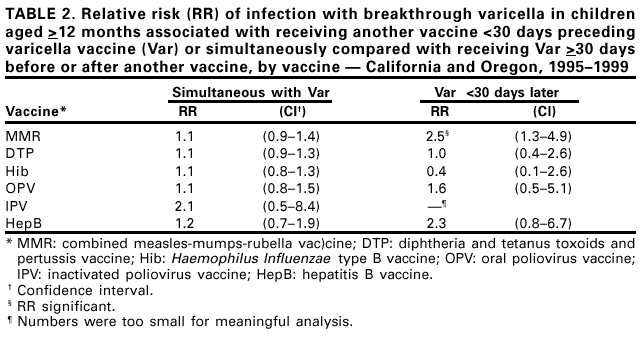
In the United States, rates of new HBV infections are highest among adults aged 40-49 years, reflecting low hepatitis B vaccination coverage among adults at risk. The most common risk factor among people with new HBV infections is injecting drugs, related to the opioid crisis.
The highest rates of chronic hepatitis B infection in the United States occur among foreign-born individuals, especially people born in Asia, the Pacific Islands, and Africa. Approximately 70% of cases in the United States are among people who were born outside of the United States. CDC developed this map of the geographic distribution of hepatitis B around the world – PDF. Other groups who have higher rates of chronic HBV infection include people who inject drugs and men who have sex with men.
Also Check: Antiviral Drugs For Hepatitis A
Many People With Hbv Dont Know They Have It
HBV infections are becoming less common in the United States. But HBV is still widespread in other parts of the world. Around 257 million people living around the world currently have HBV, and many of them dont know it. Chronic HBV is often asymptomatic, and even when it isnt, it can take months for symptoms to show up.
HBV can be transmitted through sexual contact and the use of IV drugs , and other risk factors. Although rare, there
Common And Local Adverse Events
HB vaccine
HB vaccine is well tolerated. Reactions are generally mild and transient, and include: irritability, headache, fatigue and injection site reactions in 10% or more of recipients.
HAHB vaccine
There is no increase in adverse events when HAHB vaccine is compared with HA vaccine given alone or concomitantly with HB vaccine at a different injection site. When the adult formulation of HAHB vaccine is given to children in the 2 dose schedule, there is no increase in adverse events compared with those occurring after administration of the pediatric formulation of HAHB vaccine.
DTaP-HB-IPV-Hib vaccine
Reactions are usually mild and transient, and include fever, irritability, restlessness and injection site reactions .
HBIg
Headache, diarrhea, fever, urticaria, angioedema and injection site reactions may occur.
You May Like: How Do You Contract Hepatitis A
Incidence Of Acute Hepatitis B In Australia
Newly acquired cases of hepatitis B virus infection in Australia mostly occur in young adults, through:65
- injecting drug use
- skin penetration procedures
- sexual contact
Between 2006 and 2015, the notification rate of newly acquired hepatitis B in Australia declined from 1.4 to 0.6 per 100,000 population.64
Since 2001, the rate of diagnosis of newly acquired infections has declined substantially among people aged < 29 years. The decline has been less among people aged 30 years.64,66,67 However, some new hepatitis B virus infections are asymptomatic and may go undetected.
Similar to chronic infection, the incidence of, and hospitalisation rates due to, acute hepatitis B are higher among Aboriginal and Torres Strait Islander people than the general Australian population.64
Hepatitis B vaccines are prepared using recombinant technology. After purification, the hepatitis B surface antigen protein is adsorbed onto elemental aluminium . Hepatitis B vaccines may contain up to 1% yeast proteins (but no yeast DNA
The Engerix-B and H-B-Vax II vaccines are manufactured by different processes, and the HBsAg content of equivalent doses of these 2 vaccines is different. The HBsAg content of the paediatric formulations of these 2 vaccines is half that of the corresponding manufacturers adult formulation.
Hepatitis B Vaccine Schedule
- Dosage depends on age and brand. The manufacturer’s guidance should be followed.
- Injections are given intramuscularly in the upper arm or anterolateral thigh. It should not be given into the buttock.
- The standard course of immunisation involves three injections at 0, 1 and 6 months.
- An accelerated course of 0, 1 and 2 months is possible – also for combined hepatitis A and B vaccines.
- Adults who need protection very quickly can have a schedule of 0, 7 and 21 days. Adults and children considered at very high risk should also have an accelerated schedule. After an accelerated course, a booster at one year is recommended.
- Accelerated courses may also be best for drug abusers, as they are notoriously difficult to get to complete a course.
The duration of protection provided by the hepatitis B vaccine is still unknown but is believed to be at least 20 years.
The current UK recommendation is that those who have received a primary course of immunisation, including children vaccinated according to the routine childhood schedule and individuals at high risk of exposure, do not require a reinforcing dose of HepB-containing vaccine, except in the following categories:
You May Like: Is Hepatitis C A Disease
Which Adults Should Be Vaccinated Against Hepatitis B
According to CDC recommendations, adults in the following groups are recommended to receive hepatitis B vaccine:
General
- All people age 18 years and younger.
- Anyone 19 years and older who wants to be protected from hepatitis B.
People at risk for infection by sexual exposure
- Sex partners of people who are hepatitis B surface antigen -positive.
- Sexually active people who are not in long-term, mutually monogamous relationships.
- People seeking evaluation or treatment for a sexually transmitted disease.
- Men who have sex with men.
People at risk for infection by percutaneous or permucosal exposure to blood or body fluids
- Current or recent illegal injection drug users.
- Household contacts of people who are HBsAg-positive.
- Residents and staff of facilities for developmentally challenged people.
- Healthcare and public safety workers with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids.
- People with end-stage renal disease, including predialysis, hemo-, peritoneal- and home-dialysis patients.
Others
- International travelers to regions with intermediate or high levels of endemic HBV infection.
- People with chronic liver disease.
- People with HIV infection.
- People with diabetes who are age 19 through 59 years. For those age 60 and older, clinicians should make a determination of need for
- vaccination based on their patients’ situation.
In a future issue, we will review the various hepatitis B serologic tests, who needs testing, and when they need it .