Will Community Pharmacies Be Able To Dispense These New Hepatitis C Drugs
Community pharmacists will be able to dispense the drugs. However, because these are new drugs, it may take time for pharmacies to order in sufficient stock to meet demand.
This means that patients may need to wait a couple of days after providing their script for the drugs to be available from their local pharmacy.
Prevention Of Hepatitis C In Custodial Settings:
Education about Hepatitis C and the Routes of Transmission for Inmates
- Education of inmates about the prevention and management of hepatitis C, including treatment, is a fundamental, necessary and effective preventive intervention.
Infection Control in Custodial Settings
- Each institution needs to have in place appropriate infection control procedures. Staff education and training about infection control measures in relation to blood borne viruses is an integral part of the proper application of these procedures.
Recreational Sport and Exercise
- Compliance with the Guidelines on HIV/Hepatitis and Other Blood Borne Viruses in Sport reduces the risk of exposure to blood during sport and recreational activities within custodial settings.
Provision of Bleach and Disinfectant and Education about their Use
- Provision of, and access to bleach and disinfectants is supported in custodial settings where no other safer alternatives are provided for decontaminating spills, surfaces or equipment. Education about the proper use of bleach is an essential component of its provision.
Access to Razors, Toothbrushes and Safe Barbering
Education and Counselling Related to Injecting Drug Use
- Easily accessible education and counselling about hepatitis C and injection drug use is a fundamental health promotion technique to support behaviour change. Tailoring the information to different groupsâ needs is an important component of accessibility.
Drug Treatment Programs
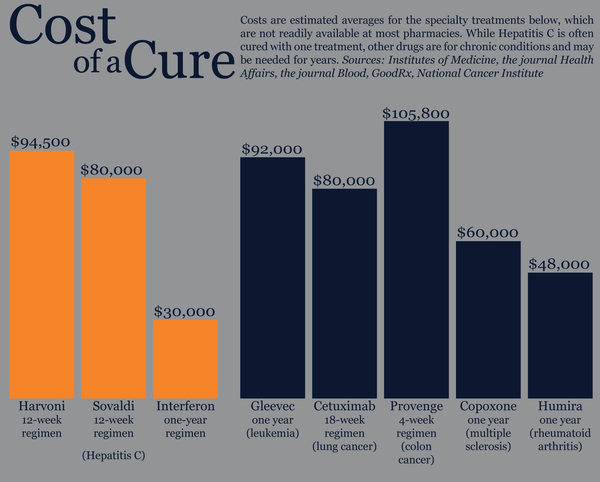
How Much Will It Cost Patients To Access The New Drugs
The Pharmaceutical Benefits Scheme listing means that hepatitis C patients will only pay the normal co-payment for the new drugs. The co-payment is currently worth $6.20 for patients with concessional healthcare cards and $38.30 for general patients without concessional healthcare cards, per drug, per month. For some patients needing three drugs, for example, the co-payment will therefore be $114.90 per month, or $18.60 per month concession.
More information on co-payment charges can be found on the Commonwealth Department of Healths website
Also Check: How Do You Get Infected With Hepatitis C
Will A Specialist Need To Be Involved
In order to prescribe, general practitioners including physicians with expertise in viral hepatitis, will be required to first consult with a gastroenterologist, hepatologist or infectious diseases physician to ensure patients with liver disease or other complex needs are appropriately referred to specialist care. A face to face consult with the specialist is not required and patients with complex needs will likely be referred to specialist care where appropriate.
Patients affected by hepatitis C with severe or advanced liver disease may still need to access the treatments under the care of a specialist – such as a gastroenterologist, hepatologist, or an infectious disease physician with experience in treating chronic hepatitis C infection.
Why Cure Hep C
Curing your hep C clears the virus from your body. It reduces liver inflammation and can help reverse fibrosis and even cirrhosis.
Live free from the worry of hep C knowing that you no longer have hep C can help you feel better about yourself. For example, you may no longer feel worried about passing hep C to other people. There has been no better time to think about hep C treatment.
Find out more about the benefits of clearing hep C call the Hepatitis Infoline.
|
Grace talks about her experience of being cured of hepatitis C with new, highly effective treatments. Theres never been a better time to be cured of hep C. |
Also Check: Hepatitis C Signs And Symptoms Cdc
Can A Transplant Cure Hepatitis C
If you develop chronic hepatitis C and it leads to liver cancer or liver failure, you may need a liver transplant. Hepatitis C is one of the most common reasons for a liver transplant.
A liver transplant removes a damaged liver and replaces it with a healthy one. However, theres a high likelihood that the hepatitis C virus will be transmitted to the new liver in time.
The virus lives in your bloodstream, not just your liver. Removing your liver wont cure the disease.
If you have active hepatitis C, continued damage to your new liver is very likely, especially if hepatitis C remains untreated.
What Is Hepatitis C
Hepatitis C is a liver disease caused by the hepatitis C virus. Hepatitis C can cause a mild illness of a few weeks or develop into a serious, lifelong, chronic illness. It is a major cause of cancer and cirrhosis of the liver, and if not treated, it can be fatal.
Hepatitis C is transmitted through exposure to small quantities of blood. People who inject drugs are at particular risk. About 30% of people infected clear the virus within 6 months without treatment. The remaining 70% develop chronic hepatitis C and for these, the risk of liver cirrhosis ranges between 15% and 30% within 20 years. 75% of those reported as living with chronic hepatitis C live in low- and middle-income countries.
Don’t Miss: Symptoms Of Advanced Hepatitis C
What About Patients With Hepatitis C Who Also Have Hepatitis B
Hepatitis B virus can flare in patients who are co-infected with hepatitis B and hepatitis C and are taking medication for hepatitis C. This has been reported as a potential risk for patients who are taking hepatitis C treatment and have underlying hepatitis B as well. The flare usually occurs within a few weeks after the patient starts taking medication for hepatitis C. Therefore, patients who have both hepatitis B and hepatitis C should be seen by a hepatitis expertbeforestarting treatment of the hepatitis C they may need to start taking hepatitis B treatment to avoid a hepatitis B flare.
Response To Antiviral Therapy
Your response to HCV therapy is evaluated during and after the completion of treatment and is defined by the following criteria:
- Rapid viral response : an undetectable viral load after four weeks of treatment
- Extended rapid viral response : an undetectable viral load at 12 weeks following the initial RVR
- Early viral response : an undetectable viral load or a 99 percent reduction in viral load by 12 weeks
- End of treatment response : an undetectable viral load achieved at 12 weeks
- Partial responder: achieves EVR, but is unable to sustain an undetectable viral load 24 weeks after therapy completion
- Null responder: unable to achieve EVR by 12 weeks
- Sustained viral response : able to sustain an undetectable viral load for 12 weeks and 24 weeks following completion of therapy
Read Also: Hepatitis C Treatment Guidelines 2017
Route Of Transmission And Treatment Response
At first, it was believed that most frequent route of transmission of HCV was blood transfusion and intravenous drug abuse. But recent epidemiological studies suggest further routes of transmission . The main route of HCV transmission is parental. However 90% intravenous drug users are at highest risk of getting HCV infection such as those who require multiple blood transfusions and blood products or those who go through major surgery . Unlike HBV, HCV infection transfer less frequently by sexual or intimate contact . Domestic contacts are also at low risk . Almost 5% HCV infections are caused by needle stick injury . 3% to 5% infants acquire HCV from infected mother by perinatal transmission . HCV is present in saliva and milk but transfer of HCV infection through breast milk has not been reported .
Community barbershops also play a key role in HCV transmission in under development countries . Some other reported risk factors of disease transmission are dental and surgical treatments, circumcision, ear piercing, tattooing and dialysis . In a study conducted on 3351 patients of HCV in Pakistan it has been documented that more than 70% hepatitis C infections are spread in hospitals by the use of same needle several times and major or minor operations that are extremely frequent in Pakistan. Globally reuse of needles is also common source of transmission . Studies show that RVR and SVR are independent of transmission routes of HCV.
What Are Current Treatments For Hepatitis C
Pegylated-interferon with ribavirin
- 40-80% cure rate
- long treatment duration
- complex management of the treatment
- administered by injection
- difficult to access in some settings.
Direct acting antivirals
- highly effective
- safer and better tolerated than existing therapies
- shorter treatment duration
- oral formulation
- simpler monitoring and laboratory requirements
- access is quite limited, mostly because of high cost
Recommended Reading: How Is Hepatitis C Passed
Sustained Viral Response: A Patient
The molecular demonstration of the absence of HCV-RNA twelve weeks after the end of a course of antiviral treatment confirms the sustained eradication of the virus. The likelihood of a late recurrence is well under 1% , and most such events are actually not recurrences but reinfections . The eradication of HCV does not generate protective immunity .
A meta-analysis of 129 studies involving a total of 34 563 patients who had undergone interferon-based treatment revealed that a sustained virological response was associated with a 62% to 84% reduction of mortality, a 68% to 79% reduction of the risk of hepatocellular carcinoma , and a 90% reduction of the risk of needing liver transplantation . As interferon-based treatment was contraindicated in patients with decompensated cirrhosis, these data are uninformative with respect to any potential clinical benefit, for these patients, of sustained viral eradication with direct antiviral agents . Initial studies have yielded clinical and laboratory evidence of improvement mainly for patients with a MELD score below 1618 points . In large-scale cohort studies, sustained viral eradication was associated both with lower liver-associated mortality and with substantially lower extrahepatic mortality . Sustained viral eradication eliminates the risk of individual transmission and is associated with a better quality of life .
HCV genotypes
Hcv Genotypes And Treatment Response
Patients with different HCV genotypes react in a different way to alpha interferon because genotype is one of the strongest prognostic aspects of sustained virological response . This clinical importance of HCV genotype was revealed by clinical studies based on interferon treatment response account . Patients show more sustained virological response when suffered from HCV genotype 2 and 3 as compared to HCV infected persons of genotype1 . Patients infected with HCV genotype 2 and 3 show 65% SVR and patients with HCV genotype 1 show 30% Sustained Virological Response . Thus genotype of patients must not be over looked when giving standard interferon therapy. Different ethnic groups respond differently to standard therapy of HCV and hence there is variation in Early Treatment Response and SVR rates .
Also Check: How Do You Get Tested For Hepatitis B
Treatment Of Patients With Impaired Renal Function/dialysis
Renal excretion is the main elimination pathway of the NS5B polymerase inhibitor sofosbuvir and its metabolites. For patients with mild to moderate renal impairment administration of sofosbuvir is safe. Since patients with severe renal dysfunction or hemodialysis have not been included during the initial phase III study trials, drug safety of sofosbuvir in these patients has not been studied in detail, and therefore sofosbuvir treatment is not recommended by the FDA and EMA label. Nevertheless, some real-world studies and preliminary data of a prospective multicenter trial show high efficacy and safety during full dose sofosbuvir treatment for patients with severe kidney dysfunc nt-related discontinuations or treatment-related serious adverse events . However, in some studies these patients also experience higher rates of anemia and worsening of kidney dysfunction . Therefore, sofosbuvir-free treatment options should be preferred if possible. Since the introduction of the pangenotypic combination of glecaprevir/pibrentasvir, a highly effective treatment of GT 2 and 3 is available, and safety and efficacy for patients with renal impairment and hemodialysis have been shown successfully . Alternatively, grazoprevir/elbasvir can be safely administered in patients with GT 1 or 4 infection and severe renal dysfunction .
Treatment After Liver Transplantation
After liver transplantation reinfection of the graft leads to fast development of liver fibrosis, and consequently the risks of organ dysfunction and graft loss are significantly increased . Thus, antiviral treatment is essential to preserve liver function and ensure transplant survival . Severe post-transplantation cholestatic hepatitis and patients with moderate to severe fibrosis need urgent initiation of antiviral treatment to prevent graft loss . In the SOLAR-1 and -2 trials patients after liver transplantation show similar SVR rates compared to nontransplanted patients with the treatment of sofosbuvir/ledipasvir plus RBV . Comparable results were achieved by the combination of sofosbuvir/velpatasvir . These results are supported by data from several real-world studies confirming the efficacy and safety of DAA treatment in the post-transplantation setting. Potential DDI between DAA and immunosuppressive drugs require special attention, in particular when using protease inhibitors. However, the combination of glecaprevir/pibrentasvir is certainly a valuable pangenotypic alternative to sofosbuvir-containing regimens, in particular for patients with impaired kidney function after liver transplantation . During DAA treatment serum levels of immunosuppressant drugs have to be closely monitored.
You May Like: Can Hepatitis C Turn Into Hiv
Current Treatment For Hepatitis C
Hepatitis C infection differs from other chronic viral infections, in that it can potentially be cured by treatment. Several medicines are available to treat individuals infected with HCV, and cure rates have steadily improved with the introduction of newer medicines.
With new drug combinations it is anticipated that it will be possible to cure approximately 90% of persons with HCV infection. These new combinations are effective against the infection in patient groups that were previously described as difficult to treat. Currently licensed treatments for HCV infection include pegylated interferon alpha , ribavirin , the protease inhibitors boceprevir, telaprevir and simeprevir and the NS5B nucleotide polymerase inhibitor sofosbuvir. Whilst interferon remains the backbone of therapy for most patient groups, interferon-free regimes are beginning to be used. Moreover, it is expected that in the next few years, a number of additional antiviral compounds will be licensed.
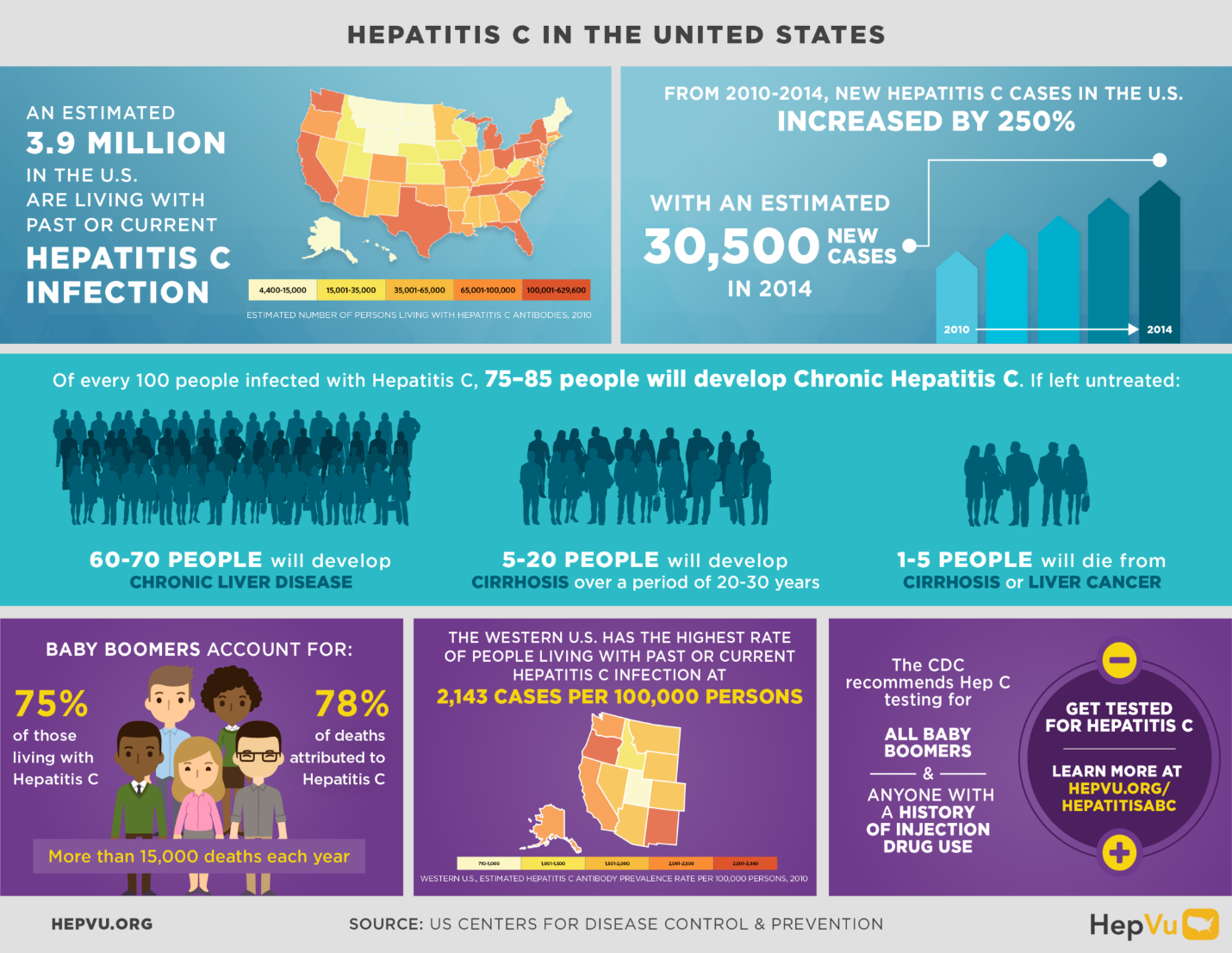
How Many Victorians Are Affected By Hepatitis C And Who Is Affected
Hepatitis C is the most common blood borne virus in Australia with approximately 230,000 people currently living with hepatitis C in Australia and around 65,000 in Victoria.
The population most at risk of acquiring hepatitis C are people who currently inject drugs including people from Aboriginal and Torres Strait Islander and culturally and linguistically diverse backgrounds, prisoners, older people, and young injectors and/or new initiates to injecting drug use.
Recommended Reading: Is Hepatitis C And Aids The Same Thing
Hepatitis C Education And Counselling
- Hepatitis C educational programs provide access to the means to protect the individual and minimise the risk of hepatitis C transmission and are necessary and effective interventions for inmates and staff in custodial settings.
Access to Educational Materials
- The ready availability of current, easy to understand information about hepatitis C in prison including its prevention and medical management supports inmates to prevent hepatitis C transmission and to seek testing and clinical assessment if they are at risk.
Purpose Developed Materials
- Resources that are designed to meet the educational needs of groups of inmates are more effective communication tools.
Peer Education
- The provision of peer education within the custodial setting is another effective and proven method to decrease the transmission of blood borne viruses.
- Cooperative development of these programs between custodial staff and peer educators maximises their success.
Access to Counselling and Support Services
- Improved access to support and counselling by a range of service providers will benefit individual inmates and the broader custodial community.
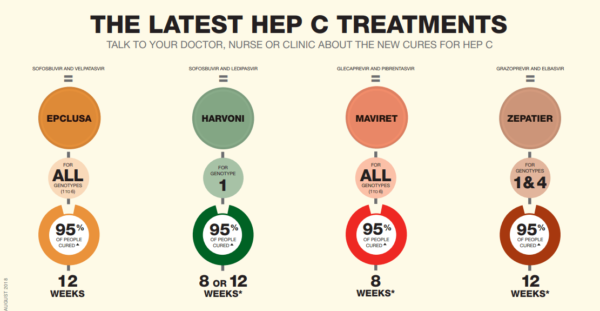
Why Are The New Hepatitis C Treatments Better
The new treatments are highly effective with a cure rate of 95-97 per cent. Treatment time is reduced to 12 weeks, drugs are tablets and there are very few side effects.
This is a major change from just a few years ago, when hepatitis C treatment time was from six to twelve months, with toxic side effects, and only a 50 percent chance of being cured.
This means that people newly diagnosed with hepatitis C, as well as those who have been living with chronic hepatitis C for many years, will now have access to a fast, effective and well-tolerated cure.
Also Check: Side Effects Of Hepatitis C
What Health Professionals Need To Know About Hepatitis C
The hepatitis C virus is transmitted primarily through parenteral exposure to infectious blood or body fluids containing infectious blood. Hepatitis C is not a vaccine-preventable infection.
Hepatitis C infection is reportable by laboratories and clinicians to local public health authorities in all provinces and territories.
Consult the national case definition for additional information.
In Canada, screening of the blood supply was implemented in 1992. This has virtually eliminated the risk of HCV transmission via transfusion. Prior to this, thousands of people contracted HCV through receiving blood or blood products.
Actions For This Page
- Recent advances in antiviral treatment have led to the development of new highly effective drugs for the treatment of all types of hepatitis C.
- The new hepatitis C treatments are sofosbuvir with ledipasvir sofosbuvir daclatasvir and ribavirin .
- These new treatments are now available on the Pharmaceuticals Benefits Scheme.
Read Also: How Does One Get Hepatitis C
Ifn Monotherapy In Acute Hepatitis C
Although the short courses of standard IFN monotherapy introduced in the 1980s by Hoofnagle et al, Davis et al, and Di Bisceglie et al led to sustained improvement in liver disease and loss of virus in less than 10% of patients, these therapies were the first to cure chronic viral hepatitis.
Jaeckel et al reported that treatment with IFN alfa-2b prevented chronic infection in 98% of a group of 44 German patients with acute hepatitis C. In this study, patients received 5 million U/day of IFN alfa-2b subcutaneously for 4 weeks and then three times per week for another 20 weeks the IFN alfa-2b was well tolerated in all patients but one.
Because it has the poorest safety profile of all the HCV antiviral agents, with few exceptions PEG-IFN is no longer recommended in combination regimens. Spontaneous resolution of acute HCV infection may occur in 15% to 50% of patients. Monitoring for spontaneous clearance for a minimum of 6 months before initiating any treatment is therefore recommended.
References
World Health Organization. Hepatitis C: fact sheet. Available at . Updated: October 2017 Accessed: January 23, 2018.
Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000 Mar 11. 355:887-91. .
Kim A. Hepatitis C virus. Ann Intern Med. 2016 Sep 6. 165 :ITC33-ITC48. .