The Goal Of Hepatitis C Therapy
Hepatitis C virus was discovered by Choo et al. in the United States of America in 1989, and it has become clear that at least 90% of patients who were diagnosed as non-A, non-B hepatitis and at least half of all patients who were diagnosed as alcoholic liver diseases have liver damages caused by HCV. It is estimated that 170 million persons around the world and between 1.5 and 2 million persons in Japan are HCV carriers. When healthy persons are infected with HCV, around 30% have an acute course and then recover, and in around 70% the HCV infection persists and progresses to chronic hepatitis. If the infection becomes chronic, the incidence of spontaneous clearance of the virus is extremely low â 0.2% annually â and hepatic fibrosis results from inflammation caused by the infection, and the disease progresses to cirrhosis and/or hepatocellular carcinoma.
Recommendations:
Factors To Consider Prior To Choosing Retreatment Regimen
For retreatment of adults with HCV genotype 2, four major factors influence the optimal regimen for retreatment, including the prior regimen the patient failed, including whether there was prior exposure to an NS5A inhibitor, the presence or absence of cirrhosis, cost or insurance considerations. The retreatment of persons with HCV genotype 2 patients who have decompensated cirrhosis, severe renal impairment , or post-liver transplantation is not addressed in this lesson.
Jsh Guidelines For The Management Of Hepatitis C Virus Infection: A 2016 Update For Genotype 1 And 2
Corresponding Author
Department of Medicine, Teikyo University School of Medicine
Corresponding Author
Department of Medicine, Teikyo University School of Medicine
Conflicts of Interest: Conflicts of interest of the âHepatitis C Treatment Guidelines â Hepatitis Treatment Guideline Preparation Committee Members
â Financial compensation
None
â¡Profits from shares
No stock owned
â¢Patent use fees
SRL Inc.
â£Speaking fees
MSD, Dainippon Sumitomo Pharma Company, Ltd., Bristol-Myers K.K., Mitsubishi Tanabe Pharma Corporation, Toray Industries, Janssen Pharmaceutica, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd.
â¤Manuscript fees
None
â¥Total amount of research fees, grants, etc. )
None
â¦Total amount of scholarship payments received in a single company or organization sharing a scholarship budget)
MSD, Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Pharmaceutical Co., Ltd.
â§Sponsored courses provided by companies, etc.
MSD, Dainippon Sumitomo Pharma Company, Ltd., Bristol-Myers, K.K., Toray Industries, Chugai Pharmaceutical Co., Ltd.
â¨Receipt of travel expenses, gifts, etc.
None
Recommended Reading: Hepatitis C Transmission Routes Cdc
Outcome Of Patients With Genotype 2 Or 3
Patients with genotype 2 or 3 showed an initial virological response rate of 100% under daily dose IFN-2a treatment. During treatment, however, three genotype 3 patients were excluded from the study due to incompliance. Thus, at that time the drop outs were virological responders. All patients who completed therapy had a complete biochemical and virological response at the end of 24-wk treatment. During follow-up, after EOT three of nine patients developed a relapse. In these patients, initial virological response first occurred after 4 wk of treatment. The remaining six patients sustainingly eradicated the virus, i.e. serum viral RNA was non-detectable for at least a 48-wk period after EOT. Data of treatment response and follow-up are shown in Figure .
Outcome of 12 HCV-infected patients with genotype 2 or 3 after the end of treatment and follow-up. Abbreviations: EOT , IFN .
Epidemiologyglobal Comparison And Resource Factors
When the epidemiology of HCV infection globally is being discussed, it is imperative to discuss northwest and eastsouth differences as well. These include a low prevalence of HCV infection in the north and west and a moderate to high prevalence in the south and eastleading to a high health-resource and financial burden on already resource-constrained countries. The main risk factor for HCV in the east is unsafe therapeutic injections, due to poor practical application of universal infection control guidelines, including reutilization of syringes, needles, or any equipment from patient to patient without adequate sterilization techniques. This affects the treatment strategies in developing countries and emphasizes the need for prevention strategies, public awareness, health education, and sensitizing health-care staff and concerned authorities in governments. On the other hand, in Western developed countries, HCV is mainly transmitted by injecting drug users sharing injection equipment. The prevalence of anti-HCV among intravenous drug users may range from 35% to 61% , and intravenous drug use accounts for 6080% of new HCV infections in the United States.
The natural history of HCV is also different in the east and west, due to specific risk factors such as alcohol use, addiction, intravenous drug use, co-infections, and superinfections. Other comorbidities and nutritional deficiencies also affect liver histology and progression of the disease.
Read Also: Hepatitis A Vaccine San Diego Free
Hyperlipidemia Diabetes Mellitus Or Ir
Lipid metabolism is intimately involved in the molecular mechanisms of the HCV infectious cycle. HCV replication influences and depends upon cholesterol uptake and efflux through different lipoprotein receptors during its entry into the hosts cells . Very low-density lipoprotein-associated proteins, including apolipoprotein B, apoE and microsomal triglyceride transfer proteins, have been shown to play a crucial role in the formation of infectious HCV particles, especially pertaining to genotype 3 . HCV can bind low-density lipoprotein receptors and lead to intracellular lipid deposition . Patients infected with genotype 3 tend to have hypocholesterolemia and hypobetalipoproteinemia, which may account for the direct effect of the virus on lipid metabolism . It appears that HCV-3 also selectively interferes with the late cholesterol pathway, a phenomenon that appears to disappear with SVR . Interestingly, high LDL levels tends to predict SVR in patients treated with interferon and ribavirin . Since LDL receptors are involved in HCV entry into hepatocytes, higher LDL levels may decrease the number of LDL receptors on the cell membrane, thus decreasing cellular infectivity .
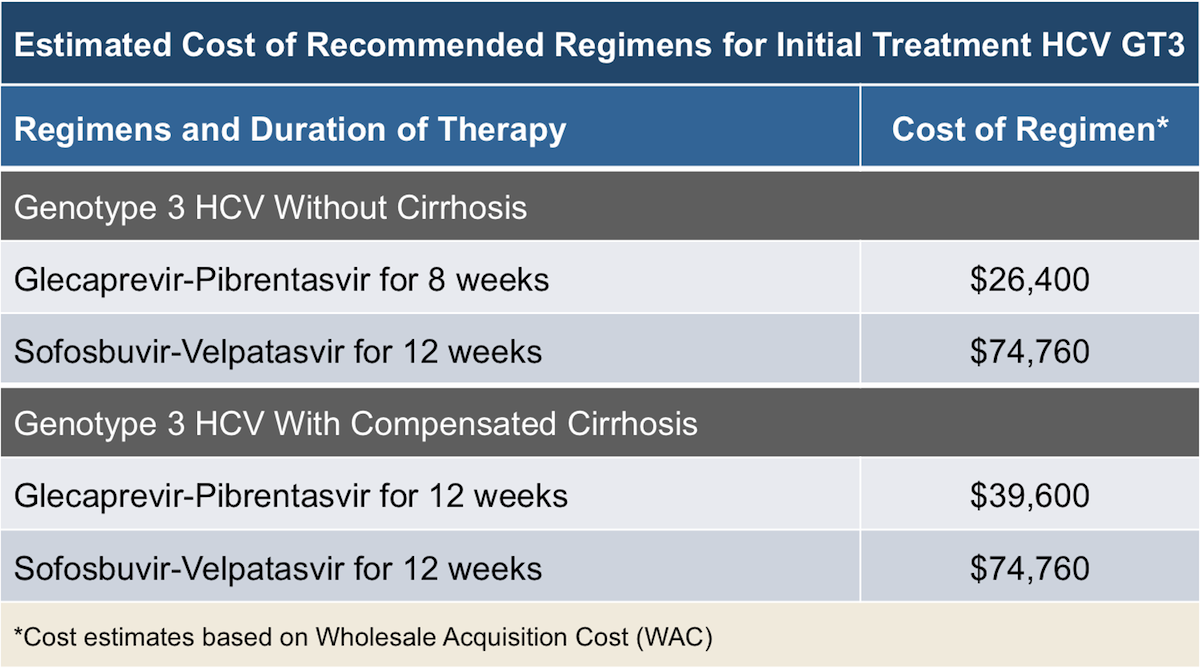
Treatment Of Hcv Genotype 3 With Compensated Cirrhosis: Sofosbuvir Plus Ribavirin
SOF plus RBV for 12 weeks is not recommended for treatment of cirrhotic patients with HCV genotype 3 infections. The overall SVR rates in naive cirrhotic patients treated for 12 weeks ranged from 21 to 34% . Two trials found that SOF plus RBV for 12 weeks for naive patients with cirrhosis resulted in SVR rates of 21% and 34% .
Extending the treatment with SOF plus RBV in this population improved the SVR rates . The Boson study included 21 naive patients with cirrhosis treated with SOF plus RBV for 16 weeks and 12 achieved SVR . Extending the treatment for 24 weeks in this population improved the SVR rates from 82 to 92% . In the Boson study, among 22 naive patients with cirrhosis treated with SOF plus RBV for 24 weeks, 18 achieved SVR . In the Valence study, 12 of 13 patients achieved SVR . SOF plus RBV for 24 weeks is recommended by EASL for treatment-naive patients with cirrhosis and by AASLD as an alternative regimen for treatment-naive patients with HCV genotype 3 infection who are IFN-ineligible .
Among treatment-experienced patients with cirrhosis and HCV genotype 3 infection treated with SOF plus RBV for 12 weeks, the SVR rates are around 20%, similar to those observed in naive patients . Only one study, the Fusion trial, assessed SOF plus RBV for 12 weeks in treatment-experienced patients with cirrhosis, including 26 patients, and only 20% achieved SVR .
Read Also: How Contagious Is Hepatitis C
Sofosbuvir Plus And Ribavirin
The combination SOF plus PegIFN/RBV for 12 weeks is recommended by EASL and AASLD for the treatment of naive or treatment-experienced patients with compensated cirrhosis and HCV genotype 3 infection . This recommendation is based in only one study, which observed an overall SVR rate of 8692% in compensated cirrhotic patients .
The Boson phase III study found SVR rates of 91% for the treatment of 23 naive patients with compensated cirrhosis with SOF plus PegIFN/RBV for 12 weeks . The treatment-experienced cirrhotic population was evaluated in two studies, which assessed the SVR rates with SOF plus PegIFN/RBV for 12 weeks . The Boson study included 35 patients and 30 achieved SVR . Lonestar, a phase II study, included 12 treatment-experienced cirrhotic patients and ten reached SVR .
Even though it was only evaluated in small cohorts, the regimen containing SOF plus PegIFN/RBV for 12 weeks presents an adequate option for patients with compensated cirrhosis however, interferon-based therapy may have some contraindications and is also associated with high rates of adverse events, especially in the cirrhotic population, justifying the ongoing search of safer and more effective therapies for these patients.
Sofosbuvir Plus Pegylated Interferon/ribavirin
The combination of SOF plus PegIFN/RBV for 12 weeks is the only interferon based therapy recommended by the EASL and AASLD guidelines for the treatment of HCV genotype 3 infection .
In naive non-cirrhotic patients, SOF plus PegIFN/RBV for 12 weeks resulted in an overall SVR of 92-100% . However, efficacy data is scarce: few patients were included in clinical trials and only three studies evaluated the SVR rates in this population. The phase II study included 25 naive non-cirrhotic patients treated with SOF plus PegIFN/RBV for 12 weeks, reaching an overall SVR rate of 92%, but no SVR data according to specific genotype is available 70033-1.). Another phase II study included 17 patients treated with SOF plus PegIFN/RBV for either 12 or 8 weeks and the overall SVR rate was 100% in both arms . The Boson phase III study included 71 naive non-cirrhotic patients with HCV genotype 3 infection treated with SOF plus PegIFN/RBV for 12 weeks, achieving an overall SVR rate of 96% .
In non-cirrhotic patients, including naive and with previous failure to PegIFN/RBV, SOF plus PegIFN/RBV for 12 weeks resulted in high SVR rates . It must be noted that non-significant differences in SVR rates were observed among naive and treatment-experienced patients, but these data need to be cautiously analyzed, since only small cohorts were included in the studies.
Recommended Reading: Is Hepatitis B Curable Or Treatable
Hepatitis C Genotype 1 Treatment Options:
Hepatitis C Genotype 1 can be treated with 12 weeks of:
In most situations, the Sofosbuvir based treatments will give a slightly higher cure rate, especially if the patient has cirrhosis of the liver or has failed previous treatment. For Genotype 1b Sofosbuvir and Daclatasvir will give a slightly higher cure rate than Harvoni.
The latest research shows that Sofosbuvir + Velpatasvir and Sofosbuvir + Daclatasvir both give essentially the same cure rates for Hep C genotype 2 and genotype 3.
What Are The Treatment Guidelines For Hepatitis C Genotype 2 Or 3 With Decompensated Cirrhosis Following Liver Transplantation
HCV genotype 2 or 3 allograft patients with decompensated cirrhosis, regardless of treatment experience
Recommended regimens
- Daily daclatasvir plus sofosbuvir with a low initial dose of ribavirin for 12 weeks
- Daily fixed-dose combination of sofosbuvir /velpatasvir with weight-based ribavirin for 12 weeks
You May Like: What Is Hepatitis C Antibody Mean
Reasons For Discontinued Therapy
Electronic patient records at Stavanger University Hospital were studied closely to reveal the reasons for discontinuing therapy. In 88.9 % cases, the therapy was completed according to the original plan. A total of 13 of 308 of treatments were discontinued because of adverse reactions and 11 of discontinuations were related to viral breakthrough, often because of noncompliance. Five treatments were discontiued for unknown reasons, while three patients discontinued treatment because of active drug abuse and two due to intercurrent illness.
Renewed 2015 Clinical Practice Guidelines For Management Of Hepatitis C By Korean Association For The Study Of The Liver What Has Been Changed Treatment Of Chronic Hepatitis C Genotype 2 And 3
Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
Correspondence to: Young Kul Jung, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, 123 Jeokgeum-ro, Danwon-gu, Ansan 15355, Korea. Tel: +82-31-412-7623, Fax: +82-31-412-5582, E-mail:
Also Check: How Is Hepatitis B Virus Transmitted
How Other Genotypes Are Treated
Treatment for genotypes 1, 3, 4, 5, and 6 also depend on a variety of factors such as viral load and the extent of liver damage. Genotypes 4 and 6 are less common, and genotypes 5 and 6 are rare in the United States.
Antiviral medications may include these drugs or combinations of them:
- daclatasvir
- ombitasvir/paritaprevir/ritonavir and dasabuvir
- simeprevir
Treatment length can vary by genotype.
If liver damage is serious enough, a liver transplant might be recommended.
Study Design And Population
This nationwide cohort study included patients infected with a nonepidemic HCV genotype treated with an interferon-free DAA regimen. Nonepidemic HCV genotypes were defined as genotypes and subtypes other than 1a/1b/2a/2b/3a/4a/4d. All laboratories performing HCV genotyping in the Netherlands were approached. All but 1 participated in the study: the Amsterdam University Medical Centers Sanquin Diagnostics, Amsterdam UMC Groningen, Groningen LUMC, Leiden Erasmus Medical Center, Rotterdam and Maastricht UMC, Maastricht.
Also Check: How To Know You Have Hepatitis
Which Treatment Works For Each Genotype
- All Genotypes: see Epclusa fact sheet
- Genotypes 1 through 4: see Sovaldi, Viekira XR and Technivie, Harvoni, Olysio fact sheets
- Genotypes 1 or 4: see Zepatier fact sheet
- Genotypes 2 or 3: see Sovaldi, Daklinza fact sheets
- Genotype 6: see Harvoni fact sheets
Ribavirin causes birth defects and miscarriage. HCV treatment regimens that include RBV should not be used by pregnant women or by male partners of pregnant women. RBV stays in a persons body for months, so women and their male partners should avoid pregnancy until six months after stopping it .
This fact sheet is current as of December 2016. It is recommended to be read alongside the Adherence and HCV Diagnostics fact sheets. Always check for updated information.
Dont Miss: What Do You Do If You Have Hepatitis C
Sofosbuvir Plus Daclatasvir With Or Without Rbv
The combination SOF plus DCV for 12 weeks is recommended by EASL and AASLD for the treatment of patients with HCV genotype 3 infection . In non-cirrhotic patients with HCV genotype 3 infection, whether naive or treatment-experienced, SOF plus DCV with or without RBV for 12 or 24 weeks resulted in an overall SVR of 80100% .
Among naive or treatment-experienced patients without cirrhosis, treatment with SOF plus DCV for 12 weeks resulted in an overall SVR of 9497% . Two studies evaluated SOF plus DCV for 12 weeks in naive or treatment-experienced non-cirrhotic patients with HCV genotype 3 infection . ALLY-3, a phase III clinical trial, included 75 naive and 34 previously treatment-experienced patients and SVR rates were, respectively, 97% and 94% . An observational study included 25 naive and treatment-experienced patients, 24 of whom achieved SVR .
A single study, ALLY-3+, evaluated the addition of RBV to SOF plus DCV for 12 or 16 weeks for HCV genotype 3 naive or treatment-experienced patients without cirrhosis, including 14 patients with advanced fibrosis, but without cirrhosis. Six patients were treated for 12 weeks and 8 patients were treated for 16 weeks with SOF plus DCV and RBV, with all of them achieving SVR12 in both the 12- and 16-week treatment arms plus sofosbuvir plus ribavirin for 12 or 16 weeks in HCV genotype 3-infected patients with advanced fibrosis or cirrhosis: The ALLY-3+ phase 3 study. AASLD Liver Meeting 2015. San Francisco, November 1317, 2015.).
Recommended Reading: How Much Cost Hepatitis C Treatment
Factors To Consider Prior To Choosing Initial Treatment Regimen
For initial treatment of persons with chronic HCV genotype 2 infection, three major factors influence the choice of regimen and duration of therapy: the presence or absence of cirrhosis, drug interactions, and medication cost and/or insurance considerations. The treatment regimens for persons with HCV genotype 2 and HIV coinfection are the same as for those for HCV genotype 2 monoinfection, with the following exceptions: persons with HIV-HCV coinfection and compensated cirrhosis should receive a 12-week course of glecaprevir-pibrentasvir versus an 8-week course with HCV monoinfection and additional drug interactions between DAAs and antiretroviral medications need to be taken into consideration.
Treatment With Sof + Dcv
SOF + DCV had been administered to 193/316 patients among whom 123 also received RBV. Treatment duration was short in 119/193 and long in 74/193 patients .
In the mITT group, SVR12 was obtained in 177/193 of those treated with SOF + DCV +/-RBV. SVR12 rates did not differ between those who received shorter versus longer treatment duration and 66/74 respectively. Nor did it differ between those who did and did not receive RBV versus 62/70 , respectively). However, among those who received short treatment there was a trend towards higher SVR12 rates in patients who received RBV compared with those who did not and 33/38 respectively p = 0.055). In further bivariate analyses, SVR12 was independent of age, gender, treatment experience, the presence of cirrhosis, the presence of decompensated liver disease and liver elasticity.
SOF+DCV was administered to 20 patients with Child Pugh B or C and SVR was achieved in 17 of these. Treatment duration was 24 weeks in 15 and 1216 weeks in 5 patients. RBV was administered to 12 patients.
Don’t Miss: Hepatitis C Can You Get Rid Of It
Screening And Linkage To Care
To increase the identification of the large proportion of persons living with undiagnosed HCV, we recommend that screening be both risk-based and target the birth cohort of individuals born from 1945 to 1975, which currently encompasses the majority of persons chronically infected with HCV in Canada .
A high proportion of Canadians with chronic HCV infection remain undiagnosed, with credible estimates ranging from 44% to 70%., The asymptomatic nature and slow progression of the infection require that individuals be identified through screening. Individuals at increased risk of infection should be tested for HCV . In addition, based on a high prevalence and low testing rate among baby boomers, a strategy of one-time screening of all individuals born between 1945 and 1975 has been shown to be cost-effective and should be implemented in Canada.
Recommended regimens and durations for patients with compensated cirrhosis who have never been treated, according to HCV genotype*
For each HCV genotype, multiple approved regimens are available. The comprehensive efficacy and safety data supporting the recommendation of each regimen for each population are provided in Appendix 1.
Genotype 1
Genotype 2
Genotype 3
Genotypes 4, 5, 6
Posttreatment follow-up
Patients who achieve sustained virologic response and do not have cirrhosis require no specific liver-related follow-up. In those with ongoing risk exposures, annual HCV RNA testing to assess for reinfection is suggested .