Hdv Coinfection With Hcv Or Denv Rescues Infectious Hdv
Next, to validate and extend the results of expression assays to a more relevant infectious context, we sought to determine if HCV-p and DENV-p particles could be produced after inoculation of live HCV or DENV to cells expressing intracellular HDV RNPs. Hence, we inoculated Huh-7.5 cells producing HDV RNAs with either cell culture-grown HCV or DENV at two different MOIs, which were set at suboptimal values in order to prevent virus-induced cell death. As control, we performed HBV infection assays in Huh-106 cells producing HDV,.
Fig. 6
Overall, these results indicated that infectious HDV particles could be assembled in cells co-infected with different viruses other than HBV, and that replication and infectivity of co-infecting virus seem not affected by HDV replication.
Zeroing In On The Hepatitis C Virus
The era of direct-acting antivirals that specifically target HCV began in 2011 with the U.S. Food and Drug Administration approval of the first protease inhibitors. These drugstelaprevir and boceprevir, along with several similar drugs approved latertargeted the HCV protease that is critical for viral replication. When used in conjunction with peginterferon and ribavirin, protease inhibitors yielded SVR rates of up to 75 percent. However, this triple therapy was accompanied by additional side effects to those already present with peginterferon and ribavirin. Nevertheless, the success of HCV-specific protease inhibitors showed that the virus had vulnerabilities that could be exploited by a well-designed and properly administered drug.
More new anti-HCV drugs were developed and tested over the next several years. These new drugs included sofosbuvir and dasabuvir, which interfered with the activity of the HCV polymerase, an enzyme that is responsible for the viral replication. Members of a second class of drugs, ledipasvir and daclatasvir, targeted the NS5A region of the virus, which makes a structural protein critical for viral replication. Many of these drugs were initially tested in conjunction with peginterferon and ribavirin, or in combination with a protease inhibitor. Generally, the results were SVR rates of at least 80 percent.
Learn More About World Hepatitis Day And How Viral Hepatitis Impacts Millions Of People Worldwide
World Hepatitis Day is recognized annually on July 28th, the birthday of Dr. Baruch Blumberg . Dr. Blumberg discovered the hepatitis B virus in 1967, and 2 years later he developed the first hepatitis B vaccine. These achievements culminated in Dr. Blumberg winning the Nobel Prize in Physiology or Medicine in 1976. Organizations around the world, including the World Health Organization and CDC, commemorate WHD to raise awareness about viral hepatitis, which impacts more than 354 million people worldwide. WHD creates an opportunity to educate people about the burden of these infections, CDCs efforts to combat viral hepatitis around the world, and actions people can take to prevent these infections.
Viral hepatitis a group of infectious diseases known as hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E affects millions of people worldwide, causing both acute and chronic liver disease. Viral hepatitis causes more than one million deaths each year. While deaths from tuberculosis and HIV have been declining, deaths from hepatitis are increasing.
The vision of CDC is to eliminate viral hepatitis in the United States and globally. CDC collaborates with international partners to help countries experiencing high rates of infection to prevent, control, and eliminate viral hepatitis.
Don’t Miss: What Is Used To Treat Hepatitis C
Interfering With Viral Replication
Viral targets
In contrast to other RNA viruses that encode RNA-dependent RNA-polymerases for replication and mRNA synthesis, HDV recruits and reprogrammes the cellular Pol II to achieve these goals. Accordingly, an important viral drug target is lacking. Nevertheless, crucial steps in the viral life cycle like the ribozyme-mediated self-cleavage of genomic and antigenomic RNA oligomers or the HDAg-dependent regulation of RNA replication and mRNA synthesis are attractive viral structures suitable for drug targeting. Inactivation of the S-HDAg could induce a selective shut down of RNA synthesis . Alternatively, abolition of the interaction of the prenylated C-terminus of L-HDAg with the cytosolic loop in the HBV S-domain by small molecules would inhibit virus release similar to LNF, which targets the corresponding host enzyme . No such drugs have been identified so far, however applying the new replication systems mentioned above will facilitate screening approaches and drug candidate identification in the future.
Cellular targets
Beside these well-characterised host factors additional approaches using siRNA or drug libraries in susceptible cell lines will allow to identify novel host factors in the future and it will be a challenging task to identify those that allow intervention and are tolerable regarding side effects.
What Is Hepatitis D
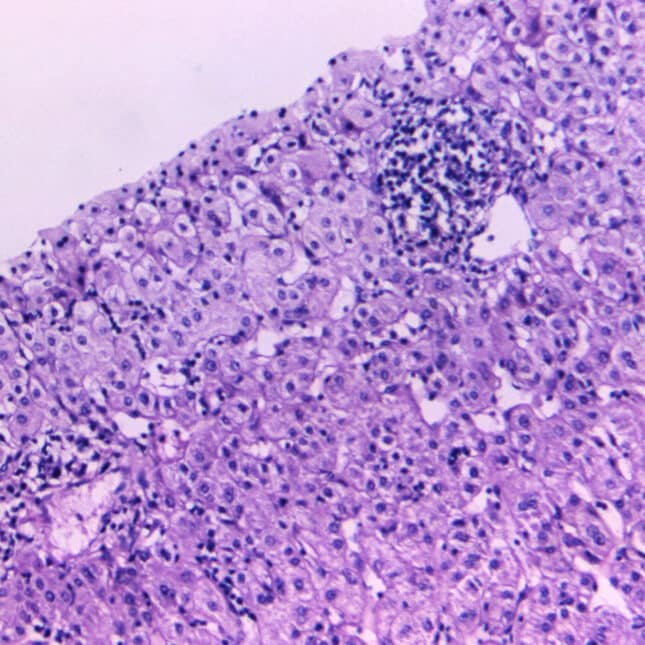
The hepatitis D virus is an RNA virus discovered in 1977 that is structurally unrelated to the hepatitis A, B or C virus. HDV causes a unique infection that requires the assistance of viral particles from hepatitis B virus to replicate and infect other hepatocytes. Its clinical course is varied and ranges from acute self-limited infection to acute fulminant liver failure. Chronic liver infection can lead to end-stage liver disease and associated complications. HDV infection occurs more commonly among adults than children. It is observed more commonly among patients with a history of intravenous drug use and in persons from the Mediterranean basin.
Recommended Reading: How Do You Know You Have Hepatitis C
Reviewhepatitis D: Thirty Years After
The key to the discovery of the Hepatitis D Virus was the description in Turin, Italy in the mid-1970s of the delta antigen and antibody in carriers of the hepatitis B surface antigen. The new antigen was first thought to be a marker of the Hepatitis B Virus and in view of its intricate true nature, it would have possibly died away as another odd antigenic subtype of HBV, like many that were described in the 1970s. Fortunately, instead, a collaboration started in 1978 between the Turin group, and the National Institute of Health and Georgetown University in the US. With American facilities and expertise this collaboration led just a year later, in 1979, to the unfolding of an unexpected and amazing chapter in virology. Experiments in chimpanzees demonstrated that the delta antigen was not a component of the HBV but of a separate defective virus requiring HBV for its infection it was named the hepatitis D virus to conform to the nomenclature of hepatitis viruses and classified within the genus Deltavirus. The animal experiments were also seminal in proposing to future clinical interpretation, the paradigm of a pathogenic infection , that could develop only in HBV-infected patients, was mainly transmitted by superinfection of HDV on chronic HBV carriers and had the ability to strongly inhibit the helper HBV. The discovery of the HDV has driven three directions of further research:
The understanding of the replicative and infectious mechanisms of the HDV.
Epidemiological Changes In The Last Two Decades In Europe
In 1983, the prevalence of anti-HD was 24.6% in carriers with liver disease in Italy. In a survey in 1987, the endemicity of HDV remained stable, with 23% of the carriers showing anti-HD and a 40% peak prevalence among cirrhotic patients. However, in two subsequent Italian surveys, the rate of anti-HD had declined to 14% in 1992 and 8.3% in 1997 . In Spain, rates of anti-HD declined from 15% in 19751985 to 7.9% in 19861992 in Taiwan, the rate of HDV superinfections diminished from 23.7% in 1983 to 4.2% in 1996. In Turkey, the prevalence of anti-HD in chronic HBsAg liver disease diminished from 31% to 11% in 19802005.
Epidemiology of hepatitis D virus in Europe in 2012. Prevalence of immigrants among HDV+. Data from Buti et al. , data from Cross et al. 2008, data from Wedemeyer and Manns 2010, data from Rizzetto and Ciancio 2012, and data from Brancaccio et al. 2014.
The issue of HDV infection has been reinvestigated in the United States. In a recent study, 50% of the chronically HBV-infected IDUs in Baltimore, MD, had anti-HD , and an 8% prevalence of anti-HD was found in 499 HBsAg carriers in northern California . In this study, HDV-positive patients had higher rates of cirrhosis than those with HBV monoinfection 69% were Caucasian non-Hispanic, 10% came from Asia and the Pacific Islands.
Recommended Reading: Dental Management Of Hepatitis Patient Ppt
Global Hepatitis Work In Other Countries
To further decrease the burden of all types of viral hepatitis, CDC also helps countries build capacity for surveillance, testing, care, and treatment and assists with development and implementation of national control and elimination programs. CDC has recently supported other countries, including Pakistan, Uzbekistan, and Tanzania.
Learn more about CDCs work to prevent hepatitis B globally.
Enhancing Healthcare Team Outcomes
Hepatitis D only occurs in patients with hepatitis B. Thus, healthcare workers, including the nurse practitioner should consider serological testing for HDV in patients with hepatitis B. This can be obtained by detection of total anti-HDV antibody followed by confirmatory staining for HDAg in liver tissues and/or measurement of serum HDV RNA. As HBV replication is suppressed in chronic HDV infection, hepatitis B e-antibodies are typically present.
As HDV depends on HBV, prevention can be achieved with hepatitis B vaccination. If the host is immune to HBV, they are subsequently protected against HDV. Patients who are at risk of contracting HDV infection should be encouraged to receive the hepatitis B vaccine.
At the moment there is no specific treatment for hepatitis D but unlike hepatitis B, the former is a benign infection.
Read Also: Is Hepatitis Curable Or Treatable
Viral Structure And Life Cycle
Hepatitis D virus viral life cycle and sites of drug target. 1. Hepatitis D virus virion attaches to the hepatocyte via interaction between hepatitis B surface antigen proteins and the sodium taurocholate cotransporting polypeptide , a multiple transmembrane transporter. 2. HDV ribonucleoprotein is translocated to nucleus mediated by the hepatitis D antigen . 3. HDV genome replication occurs via a rolling-circle mechanism. 4. HDV antigenome is transported out of the nucleus to the endoplasmic reticulum . 5. HDV antigenome is translated in the ER into small HDAg and large HDAg . 6. L-HDAg undergoes prenylation prior to assembly. 7. S-HDAg is transported back to the nucleus where it supports HDV replication. 8. New HDAg molecules are associated with new transcripts of genomic RNA to form new RNPs that are exported to the cytoplasm. 9. New HDV RNP associates with hepatitis B virus envelop proteins and assembled into HDV virions. 10. Completed HDV virions are released from the hepatocyte via the trans-Golgi network.
Finally, once the RNP interacts with the envelop protein of HBV and the HDV is assembled, the HDV virion is now ready for release. The HDV virion is released via the trans-Golgi network, where it can go on to infect other hepatocytes. However, the exact mechanism of HDV-virion release remains unknown .
Stories From The Field: Cdc Collaborates With The Country Of Georgia
CDC representatives meet with Dr. Amiran Gamkrelidze and other leadership at the National Center for Disease Control and Public Health in Georgia to discuss the Hepatitis C Elimination Program.
Beginning in 2015, CDC partnered with the country of Georgia to launch the first Hepatitis C Elimination Program in the world. In 2019, Georgia was designated the first-ever EASL International Liver Foundation Center of Excellenceexternal icon in viral hepatitis elimination.
In June 2021, CDC and the National Center for Disease Control and Public Health, Tbilisi, Georgia conducted training and launched the second nationwide hepatitis B, hepatitis C, and COVID-19 serosurvey. The field work was completed in October 2021 and results show a 67% reduction in HCV infection since 2015. Information from this survey will guide ongoing efforts to meet viral hepatitis elimination targets. CDCs international work helps reduce disease burden globally, including for overseas travelers and those migrating to the United States.
CDC works closely with the Infectious Diseases, AIDS & Clinical Research Hospital in Tbilisi, Georgia.
Also Check: Who Need Hepatitis B Vaccine
The Future Of Hepatitis C Therapy
With such high rates of success with current treatments, it may seem like the hepatitis C story is in its final chapters, but it is not over yet. A vaccine against hepatitis C would cause the prevalence of the disease to plummet, but efforts to produce a vaccine, while still under way, have not yet been successful. While hepatitis A and B have vaccines, the hepatitis C virus is more variable than either of these viruses, which, along with other factors, complicates vaccine development efforts. Additionally, the current drugs show great promise, but the costs of the more successful FDA-approved DAA treatments are extremely high, which present a significant obstacle to many with the disease. But the research has come a long way. From the early investigations into a mysterious new virus, to the identification of the culprit, and the rigorous work to develop an effective treatmentthe story of hepatitis C is definitely a thriller.
Hdv Assembled With Heterologous Envelope Gps Is Infectious
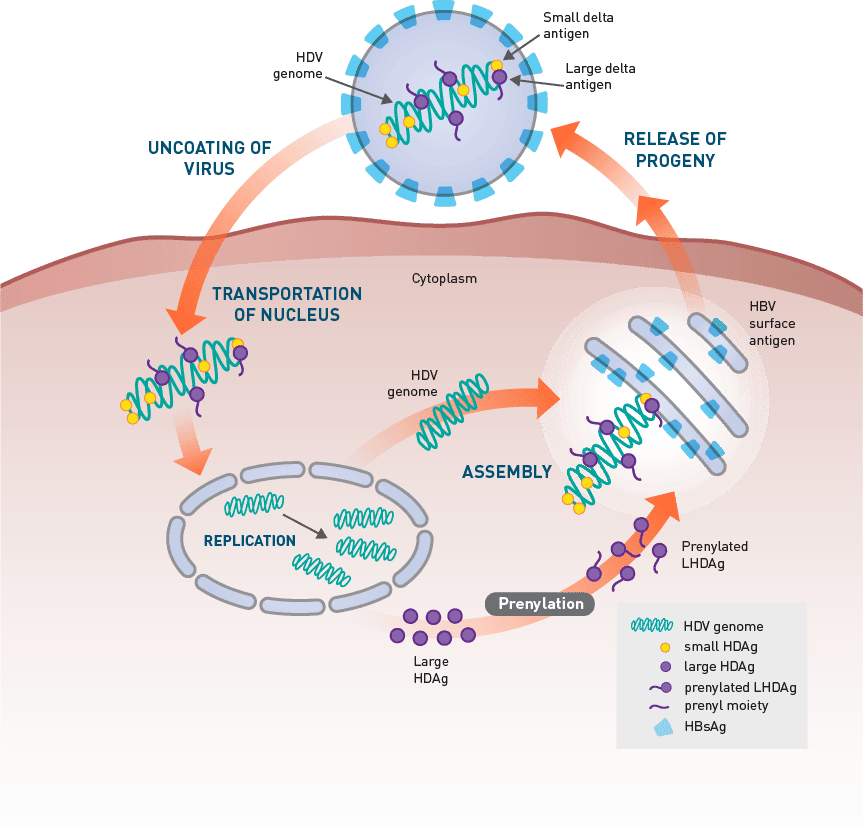
Fig. 2
Next, to demonstrate that HDV RNA was transmitted by a bona fide HDV infectious process, we incubated producer cells with Lonafarnib, an inhibitor of prenylation that prevents HDV assembly,, which requires RNP targeting to the ER membrane by farnesylation of L-HDAg. We found that Lonafarnib could readily inhibit production of HBV GP-coated HDV, VSV-p, and HCV-p particles and hence, transmission and replication of HDV RNA in inoculated cells . These results indicated that farnesyl-mediated targeting to ER or other cell membranes is required for assembly of VSV-p and HCV-p particles, suggesting that they share with HDV the same early steps, leading to production of infectious particles. Through time-course analysis, we found that cells inoculated with VSV-p and HCV-p particles accumulated over time post infection both gRNA and agRNA , which indicated that HDV RNAs could be amplified in a typical manner following entry into cells. We show that this correlated with accumulation of genomic-size HDV RNA as well as of S-HDAg and L-HDAg proteins at similar levels and/or ratios than for HBV GP-coated HDV particles, which indicated that full-sized HDV genomes were replicated and translated in infected cells. Altogether, these results demonstrated that HDV particles coated with the envelope GPs of VSV and HCV induce functional entry into cells and, hence, are infectious.
Fig. 3Fig. 4Fig. 5
Don’t Miss: Hepatitis B Test Kit Walgreens
What Are The Different Types Of Hepatitis Occurring Around The World
The five hepatitis viruses hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E are distinct and can spread in different ways, affect different populations, and result in different health outcomes.
Hepatitis A is a vaccine-preventable liver infection caused by the hepatitis A virus . HAV is found in the stool and blood of people who are infected. Hepatitis A is very contagious. It is spread when someone unknowingly ingests the virus even in microscopic amounts through close personal contact with an infected person or through eating contaminated food or drink. Symptoms of hepatitis A can last up to 2 months and include fatigue, nausea, stomach pain, and jaundice. Most people with hepatitis A do not have long-lasting illness. The best way to prevent hepatitis A is to get vaccinated.
Links with this icon indicate that you are leaving the CDC website.
- The Centers for Disease Control and Prevention cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website’s privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance on other federal or private website.
We take your privacy seriously. You can review and change the way we collect information below.
Story Of Discovery: Hepatitis C: From Non
The story of hepatitis C from discovery to cure is very much like the plot of a good mystery novel. It begins with a puzzling who-done-it, followed by a lengthy hunt for the suspect, and, finally, rigorous efforts to subdue the perpetrator. Many of these efforts were spearheaded by the NIDDK, and, although the narrative is not quite finished, the battle against hepatitis C is evolving into one of the biggest modern success stories in scientific research.
Read Also: Hepatitis C False Positive Antibody
Hcv/hdv Coinfection Can Disseminate In Vivo
We then sought to demonstrate that HCV could propagate HDV RNPs in vivo. We generated cohorts of liver-humanized mice derived from the FRG mouse model . We retained the animals that displayed > 15mg/mL of human serum albumin , which corresponded to 4070% of human hepatocytes in the liver. In agreement with previous reports,, these animals supported HBV and HCV infection for several months . In contrast, inoculation of HuHep-mice with helper-free HDV, i.e., HDV particles produced with HBV GP-expression plasmid , did not lead to HDV viremia, as shown by RT-qPCR values in infected animal sera that were identical to those detected in the non-infected HuHep-mice control group . The other groups of HuHep-mice were inoculated with either helper-free HDV followed by HCV 4 weeks later , HCV followed by helper-free HDV , or both HCV and helper-free HDV simultaneously . HDV RNAs were detected in animals of the three latter groups within a few weeks after inoculation. All HCV-positive animals of these groups were also positive for HDV and secreted HDV RNA of genomic size was detected in the sera . We obtained qualitatively comparable results in HuHep-mice co-infected with HDV and HBV . Of note, similar results were obtained in another cohort of HuHep-mice in which HDV was inoculated 1 week after HCV . Altogether, these results indicated that HDV can be propagated in vivo by different virus types, including HCV.
Fig. 7