Duke Surgeons Expertise With Hai Pumps
Drs. Lidsky and Allen and their Duke colleagues have implanted more than 60 pumps in people with liver metastases from colorectal cancer since they started offering the procedure in conjunction with whole-body chemotherapy in late 2018. In many cases, they also work alongside Duke surgical oncologist Sabino Zani, MD, who uses minimally invasive techniques, including robotic surgical approaches to implant the pump. The minimally invasive approach, which requires smaller incisions than traditional surgery, allows people to spend less time in the hospital, and recover faster.
Rationale Of Hepatic Arterial Infusion Chemotherapy
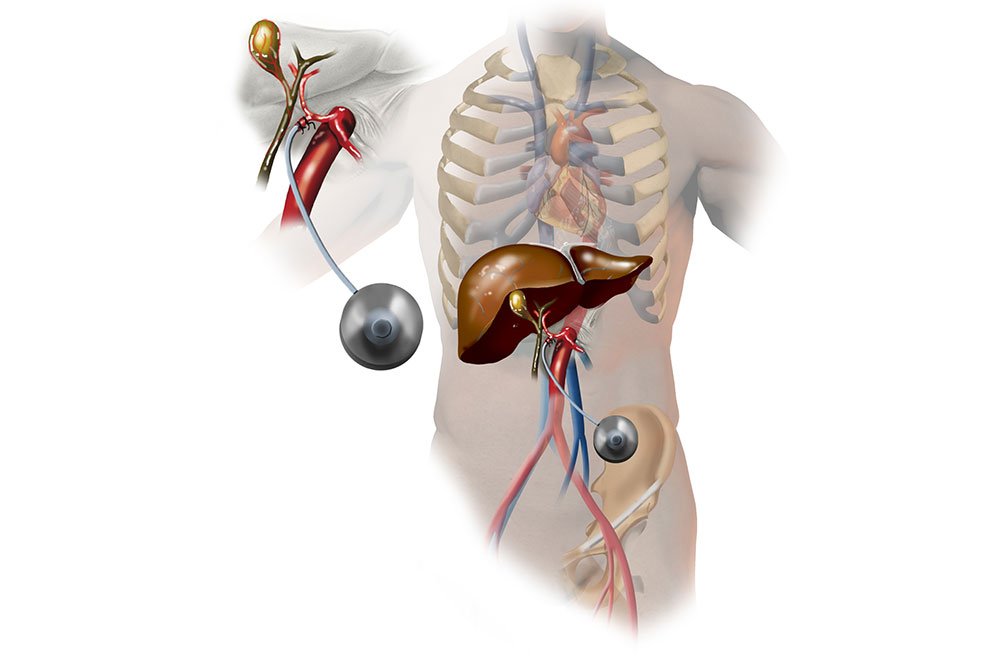
There may be a stepwise pattern of metastatic spread in CRC with the cancer originating in the colon or rectum and spreading to the liver via the portal veins. The rationale for HAI chemotherapy is based on the dual blood supply in the liver, with liver metastases greater than 1 cm in diameter deriving blood supply from the hepatic artery, whereas normal hepatocytes are perfused by the portal vein . HAI chemotherapy is administered in the gastroduodenal artery by a surgically implanted pump, a hepatic arterial port, or through a percutaneously placed catheter connected to an external pump.
What Happens On The Day Of Surgery
- Follow the instructions exactly about when to stop eating and drinking. If you don’t, your surgery may be canceled. If your doctor told you to take your medicines on the day of surgery, take them with only a sip of water.
- Follow your doctor’s instructions about when to bathe or shower before your surgery. Do not apply lotions, perfumes, deodorants, or nail polish.
- Do not shave the surgical site yourself.
- Take off all jewelry and piercings. And take out contact lenses, if you wear them.
Read Also: Hepatitis C Non Reactive Test Result
Hai Of Biologic Agents
HAI has been examined as a means of regional delivery of gene therapy for liver metastases. Based on the fact that the p53 gene is frequently defective or deleted in CRC and other cancers, two approaches using recombinant adenoviruses are being investigated. The first uses a replication-incompetent virus encoding wild-type p53 to infect cancer cells and replace the deficient gene product. HAI of one such adenoviral vector was well tolerated in a phase I study and achieved significant transgene expression at higher doses this vector is currently being studied further, both alone and in combination with HAI of FUDR . A second approach uses replication-selective viruses lacking the E1B 55-kDa gene as a means of targeted oncolysis. This gene product binds to p53 and inhibits it, allowing for viral replication and cytotoxicity. A virus lacking the E1B 55-kDa gene is unable to inhibit wild-type p53, and therefore, selectively replicates in p53-deficient cancer cells while sparing normal cells. One such virus, Onyx-15 was administered via HAI to 11 patients with refractory metastatic gastrointestinal cancers to the liver, with no dose-limiting toxicity and documented replication in vivo . Further studies of these approaches are warranted.
Hepatic Arterial Infusion As Adjuvant Therapy After Hepatic Resection
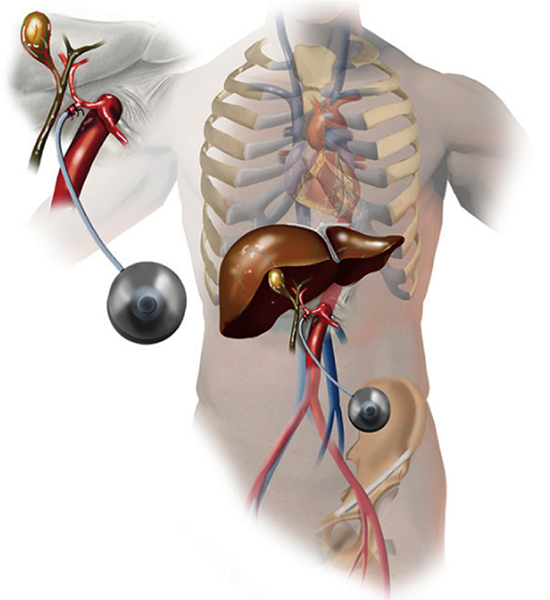
HAI chemotherapy can be used in conjunction with surgery and systemic chemotherapy to target metastatic disease from CRC to the liver. Hepatic resection of colorectal liver metastases has improved 5-year survival rates . Nearly 70% of patients develop recurrent disease after hepatic resection, usually within 2 years, and 30-50% develop isolated hepatic metastases . Adjuvant regional therapy with HAI can target residual micrometastatic disease in the liver, in conjunction with systemic therapy to reduce systemic recurrence. Several randomized studies have compared HAI plus systemic therapy versus systemic therapy alone.
A randomized trial at Memorial Sloan Kettering Cancer Center compared 156 patients who were resected and then randomized to receive HAI FUDR/Dex combined with systemic intravenous 5-FU/LV and 5-FU/LV alone . The endpoint was 2-year survival which was significantly improved with HAI FUDR/Dex plus intravenous 5-FU/LV versus 72% for 5-FU/LV alone . In addition, there was an excellent 2-year hepatic disease-free survival for those who received HAI FUDR/Dex + intravenous 5-FU/LV versus systemic therapy alone. In 2005, updated results were published and showed a 10-year survival rate of 41% in the group who received HAI FUDR/Dex plus intravenous 5-FU/LV compared to 27.2% in the group who received intravenous 5-FU/LV alone, as well as a longer overall progression-free survival .
Table 1
Randomized studies after liver resection: hepatic disease-free survival
Fig. 3
Read Also: Royal Canin Veterinary Diet Hepatic Formula Dry Dog Food
Improving Patient Access And Outcomes
While hepatic arterial infusion has only been available at a handful of centers, Dr. Lidsky hopes that will change as a result of the FDA approval. He co-leads a consortium of programs from around the world to perform studies that further demonstrate the HAI pumps effectiveness and to better identify the right candidates for the procedure. The goals of the consortium are to further improve HAI outcomes, help launch new HAI centers, and make the pump more widely available to patients, he said.
Molecular Markers And Outcomes Of Liver Resection And Hai Therapy
Testing for molecular markers is being increasingly used to guide selection of therapy, predict response to targeted therapies, and to offer more exact prognosis. Current NCCN guidelines recommend all patients with CRC have tissue genotyped for RAS and BRAF mutations, and mismatch repair DNA testing or microsatellite instability immunohistochemistry. About 2040% of CRC patients carry a mutation in the RAS gene and about 10% carry a BRAF mutation . MSI due to defective MMR is present in about 15% of all CRCs .
RAS and BRAF mutations have been known to independently predict poor prognosis in metastatic CRC and are both associated with resistance to EGFR targeted therapies. Since the discovery and usage of anti-EGFR antibodies, cetuximab and panitumumab approved in 2004 and 2007 respectively, RAS testing was the earliest molecular marker to became part of NCCN and American Society of Clinical Oncology guidelines in 2009 . There is stronger evidence supporting the negative prognostic significance of RAS than BRAF, due to the higher prevalence of RAS in CRC . A 2011 study showed increased rate of peritoneal and distant lymph node metastases with BRAF-mutant tumors, and lower OS . Another large study at MSKCC showed that BRAF-mutant CRC less commonly presents with disease limited to the liver, and also showed shorter OS, 20 vs. 47 months when compared to BRAF wild-type .
Read Also: Can Hepatitis B Be Cured With Antibiotics
Duke Surgeons Implant First Fda
Michael Lidsky, MD, and Justin Barr, MD, work to implant an HAI pump into a patient’s abdominal wall.
A chemotherapy infusion pump is giving more time to people whose metastatic colorectal cancer has spread to the liver and who may have been told they are out of options. Duke Health surgical oncologists were the first to implant the hepatic artery infusion pump following its FDA approval. They are among the few U.S. surgeons with the training and expertise to offer this highly specialized treatment.
Most people are looking to live as long as possible with the best quality of life, said Michael Lidsky, MD, a Duke Health surgical oncologist who performs the procedure. Hepatic artery infusion can give them that. And, now that the pump is approved for use by the FDA, we hope more people will get access to it and the benefits it provides.
Orr In The Matched Population
Table 2 lists the objective response in intrahepatic and extrahepatic lesions separately. For intrahepatic lesions, five CR and 36 PR were observed in patients treated with HAI. The intrahepatic ORR was 66.1% in the HAI group, which was significantly higher than 22.6% in the non-HAI group . In the HAI group, 44 patients had evaluable extrahepatic lesions and 11 patients achieved PR. In the non-HAI group the extrahepatic ORR was 28.9% . There was no significant difference in extrahepatic ORR . Figure 1 demonstrates CT and DSA images of a patient who achieved PR with SCT and HAI.
Table 2 Objective response rate n .
Figure 1 A patient with pancreatic cancer and synchronous liver metastases, who was treated with systemic GC HAI FUDR. CT images showing the presence of pancreatic cancer and synchronous liver metastases at baseline. CT images show primary cancer and liver metastases are shrinking at 6 months. images show sustained response of liver metastases and primary cancer. Hepatic arteriography shows HAI pump functionality at 12 months .
Thirty-six patients in the HAI group and 39 patients in the non-HAI group underwent second-line SCT . In the HAI group, HAI was continuously administrated for median four cycles in combination with second-line SCT in 17 patients who developed extrahepatic disease progression but had disease control in the liver. In addition, eight patients received HAI in second-line treatment in the non-HAI group.
Don’t Miss: How Does Hepatitis C Affect The Liver
Hai And Systemic Fluoropyrimidines
A study of 44 patients with unresectable liver metastases compared HAI of FUDR with concurrent HAI and i.v. FUDR. A lower rate of extrahepatic spread was seen in the combination group , but response rates, toxicities, and survival were similar in the two arms . Another single-arm study treated 40 patients with sequential HAI of FUDR and i.v. 5-FU/LV. The response rate was 62%, with a median TTP of 9 months and a 45% incidence of extrahepatic progression . Of note, CRC metastases to the lung, the most common site of extrahepatic spread in patients treated with HAI therapy, have been shown to express higher levels of TS compared with hepatic metastases . This is clinically significant, because high TS expression has been reported to predict resistance to 5-FU therapy , implying that combinations of HAI therapy with fluoropyrimidines may have limited efficacy in preventing extrahepatic disease.
Hepatic Artery Infusion Pain
Cytotoxic infusions into the hepatic artery are often associated with the development of a diffuse abdominal pain . Continuous infusions can lead to persistent pain. In some patients, the pain is due to the development of gastric ulceration or erosions , or cholangitis . If the latter complications do not occur, the pain usually resolves with discontinuation of the infusion. A dose relationship is suggested by the observation that some patients will comfortably tolerate reinitiating of the infusion at a lower dose
Aiman Ghufran, Michael R. Lucey, in, 2018
Don’t Miss: Is Hiv The Cause Of Hepatitis B
Hepatic Artery Infusion Pump Chemotherapy: A New And Promising Way To Treat Metastasized Colorectal Cancer
In its later stages, colorectal cancer can spread, or metastasize, to other organs in the body. One common site of metastasis is the liver, with about half of colorectal cancer patients developing liver metastases at some point in their cancer journey.
Treating a metastasis to the liver can be complicated. The ultimate goal is to remove the tumor surgically, but only about 1520 percent of patients present with tumors that can be surgically removed when they are first discovered. This is because they are often too big or too numerous to be safely taken out at the start of treatment.
Chemotherapy can help shrink some large liver tumors so they can be removed, but unfortunately, traditional chemotherapy doesnt work for every patient, said Jason A. Castellanos, MD, MS, a surgical oncologist at Fox Chase Cancer Center.
What can shrink colorectal liver metastases more effectively is something called a hepatic arterial infusion pumpa new and advanced method of delivering chemotherapy directly to these tumors.
Hepatic arterial infusion pump chemotherapy is a way to increase chemotherapy dosage to the liver for an improved response, Castellanos said.
The pump can help make surgery an option for more patients, which is the only treatment option that offers a chance at long-term cure.
Identification And Characteristics Of Study Patients
From July 2018 to December 2019, 160 patients with HCC who received HPL or PL were screened: 56 patients received previous surgery, interventional therapies, tyrosine kinase inhibitors or immune-targeted therapies 23 patients participated in other treatments during HPL or PL 8 patients were classified with a tumor grade of BCLC/A 1 patient was classified as CP C and 2 patients had missing sections in their medical records. Finally, a total of 70 patients who met the criteria were included in the study, and the patients were divided into the HPL group and PL group . The patient characterization process is shown in Figure 1. Of note, the treatment of PD-1 inhibitors plus lenvatinib was available since July 2018 at our center.
Figure 1 Flow diagram summarizing the disposition process of patients.
The clinical characteristics and treatment of patients are summarized in Table 1. Most patients were classified into CP A and BCLC/C . Two groups were comparable in the clinical characteristics, liver function, and tumor characteristics. A higher proportion of patients in the PL group had extrahepatic metastasis compared to the HPL group , but the difference was not statistically significant . In the HPL group, the cycles of PD-1 inhibitors plus lenvatinib ranged from 2 to 12, with a median of 5. While in the PL group, the cycles of PD-1 inhibitors plus lenvatinib ranged from 2 to 9, with a median of 4. The PD-1 inhibitor categories in each group are summarized in Table S1.
Also Check: Hepatitis C Genotype 3 Treatment
Which Chemotherapy Drugs Are Used For Liver Cancer
Unfortunately, most chemo drugs do not have a great effect on liver cancer. Recent advances have shown that a combination of drugs may be more helpful than using just a single chemo drug. But even these combinations of drugs shrink only a small number of tumors, and the responses often do not last long. And most studies show systemic chemo has not helped patients live longer.
The most common chemotherapy drugs for treating liver cancer include:
- Gemcitabine
- Capecitabine
- Mitoxantrone
Sometimes, combinations of 2 or 3 of these drugs are used. GEMOX is one option for people who are fairly healthy and may tolerate more than one drug. 5-FU based chemotherapy, for example with FOLFOX , is another option for people with bad liver disease.
Why Is The Chemotherapy Injected Into The Hepatic Artery
The normal liver gets its blood supply from two sources: the portal vein and the hepatic artery . Primary liver cancer, also known as hepatoma or hepatocellular carcinoma gets its blood exclusively from the hepatic artery. These techniques can also be used to treat secondary, or metastatic liver cancer, which is cancer that spread to the liver from other primary sites. These metastases also draw their blood supply from the hepatic arteries. This discussion will focus on primary liver cancer. Making use of this pattern of blood supply, investigators have delivered chemotherapy agents selectively through the hepatic artery directly to the HCC tumor. The theoretical advantage is that higher concentrations of the agents can be delivered to the cancer. The technique takes advantage of the concept of extraction: toxicity can be reduced by relying on the liver to extract or break down some of the chemotherapy after the tumor has been exposed to it before the chemotherapy gets through the liver into the systemic circulation.
Read Also: Hepatitis B E Antibody Reactive Means
Possible Side Effects Of Chemotherapy For Liver Cancer
Chemo drugs attack cells that are dividing quickly, which is why they work against cancer cells. But other cells in the body, such as those in the bone marrow, the lining of the mouth and intestines, and the hair follicles, also divide quickly. These cells are also likely to be affected by chemo, which can lead to side effects.
The side effects of chemo depend on the type and dose of drugs given and the length of time they are taken. Common side effects include:
- Hair loss
- Increased chance of infections
- Easy bruising or bleeding
- Fatigue
These side effects usually dont last long and go away after treatment is finished. There are often ways to lessen them. For example, drugs can be given to help prevent or reduce nausea and vomiting. Be sure to ask your doctor or nurse about drugs to help reduce side effects.
Along with the possible side effects in the list above, some drugs may have their own specific side effects. Ask your health care team what you can expect.
You should report any side effects you notice while getting chemotherapy to your medical team so that you can be treated promptly. In some cases, the doses of the chemotherapy drugs may need to be reduced or treatment may need to be delayed or stopped to prevent side effects from getting worse.
How To Prepare For Hepatic Arterial Infusion
Your surgeon will give you specific guidance on how to prepare for your cancer surgery.
These guidelines may include the following:
- Complete any necessary preoperative testing at least a week before your surgery date. These might include blood and urine tests, a chest x-ray, an EKG, and others as needed.
- Stop taking aspirin, blood thinners, or anti-inflammatory drugs 10 days before your surgery. Your doctor will let you know if and when you should stop vitamins or other supplements.
- Stop eating and drinking at least eight hours before your surgery. Your doctor may let you take medicine with a sip of water the morning of surgery. If you have diabetes, ask whether you should take your diabetes medications on surgery day.
You May Like: How Do You Get Hepatitis C
Criteria For Defining Ductopenia
Bile ducts and hepatic artery branches are detectable in 93 ± 6% and 91 ± 7% of portal tracts, respectively, of normal livers. A portal tract can be defined as a focus within the parenchyma containing connective tissue and at least two luminal structures embedded in the connective tissue mesenchyme, each with a continuous connective tissue circumference. Although lower figures for bile ducts/portal tract in normal livers have been cited, two standard deviations from normal are reached when < 80% of portal tracts contain bile ducts. Arterial loss is considered present when < 77% of portal tracts contain hepatic artery branches. Ductopenia has also been defined by at least one unpaired artery in > 10% of all portal tracts or two unpaired arteries in different portal tracts. Unpaired arteries were defined as arteries without an accompanying bile duct within a radius of 10 hepatic artery diameters from the edge of the artery.
Late CR, however, can cause both bile duct and arterial loss, which can present difficulties when trying to apply the earlier-mentioned algorithms. Portal tract recognition should be based primarily on the location of the putative structure cholestasis in CR is always centrilobular.
Nathan I Cherny, in, 2003