Sofosbuvir Plus Daclatasvir With Or Without Rbv
The combination SOF plus DCV for 12 weeks is recommended by EASL and AASLD for the treatment of patients with HCV genotype 3 infection . In non-cirrhotic patients with HCV genotype 3 infection, whether naive or treatment-experienced, SOF plus DCV with or without RBV for 12 or 24 weeks resulted in an overall SVR of 80100% .
Among naive or treatment-experienced patients without cirrhosis, treatment with SOF plus DCV for 12 weeks resulted in an overall SVR of 9497% . Two studies evaluated SOF plus DCV for 12 weeks in naive or treatment-experienced non-cirrhotic patients with HCV genotype 3 infection . ALLY-3, a phase III clinical trial, included 75 naive and 34 previously treatment-experienced patients and SVR rates were, respectively, 97% and 94% . An observational study included 25 naive and treatment-experienced patients, 24 of whom achieved SVR .
A single study, ALLY-3+, evaluated the addition of RBV to SOF plus DCV for 12 or 16 weeks for HCV genotype 3 naive or treatment-experienced patients without cirrhosis, including 14 patients with advanced fibrosis, but without cirrhosis. Six patients were treated for 12 weeks and 8 patients were treated for 16 weeks with SOF plus DCV and RBV, with all of them achieving SVR12 in both the 12- and 16-week treatment arms plus sofosbuvir plus ribavirin for 12 or 16 weeks in HCV genotype 3-infected patients with advanced fibrosis or cirrhosis: The ALLY-3+ phase 3 study. AASLD Liver Meeting 2015. San Francisco, November 1317, 2015.).
Hepatitis C With Decompensating Cirrhosis
Until recently, doctors considered a liver transplant to be the only effective treatment for decompensating cirrhosis.
However, a recent small-scale study found that a course of direct-acting antiviral medication may improve some peoples liver function enough to take them off the waiting list for a liver transplant. People with liver disease that was less severe had a higher likelihood of removal from the list.
However, recent Canadian guidelines warn that certain antiviral drugs may potentially be dangerous for people with severe decompensating cirrhosis. This is because the liver is less able to filter out toxic waste, meaning that the antiviral drugs could accumulate to toxic levels. Doctors must weigh up the benefits against the risks.
When a person is waiting for a liver transplant, a doctor will assess whether or not to pause antiviral treatment.
Also Check: Can Hepatitis Turn Into Hiv
Significance Of The Study
What is already known about this subject?
-
Hepatitis C virus genotype-3a is now the most prevalent HCV subtype in the UK and South Asia.
-
Prophylactic and therapeutic vaccines are currently in development against genotype-1 infection. However, the T cell targets in genotype-3 infection are currently unknown.
-
Interferon therapy has immunomodulatory properties HCV genotype-3a is more responsive to interferon based therapies than HCV genotype-1. The effects of interferon on genotype-3a T cell immunity are not known.
-
IL28B linked polymorphisms are associated with sustained virological response rates in HCV genotype-1 but not genotype-3a infections therefore, alternative mechanisms that explain this observation should be explored.
What are the new findings?
-
In contrast to genotype-1 infection, genotype-3a-specific CD8 T cell responses commonly target the non-structural hepatitis C virus proteins in chronic disease.
-
In chronic genotype-3a infection, T helper responses target a dominant HCV core protein.
-
T cell targets identified during chronic infection may have a limited role in protective immunity since these differ from those found in spontaneously resolved infection where CD4 T cells targeting non-structural proteins dominate.
-
Paradoxically, genotype-3a-specific T cell responses and total lymphocyte counts decline during interferon treatment in association with a sustained virological response.
How might it impact on clinical practice in the foreseeable future?
Recommended Reading: Can You Die With Hepatitis C
How Is Viral Hepatitis Prevented
Prevention of hepatitis involves measures to avoid exposure to the viruses, using immunoglobulin in the event of exposure, and vaccines. Administration of immunoglobulin is called passive protection because antibodies from patients who have had viral hepatitis are given to the patient. Vaccination is called active protection because killed viruses or non-infectious components of viruses are given to stimulate the body to produce its own antibodies.
Avoidance of exposure to viruses
Use of immunoglobulins
Immune serum globulin is human serum that contains antibodies to hepatitis A. ISG can be administered to prevent infection in individuals who have been exposed to hepatitis A. ISG works immediately upon administration, and the duration of protection is several months. ISG usually is given to travelers to regions of the world where there are high rates of hepatitis A infection and to close or household contacts of patients with hepatitis A infection. ISG is safe with few side effects.
Hepatitis A
Individuals at increased risk of acquiring hepatitis A are:
Some local health authorities or private companies may require hepatitis A vaccination for food handlers.
Hepatitis B
Genotypes Of Hepatitis C
Which genotype of hepatitis C somebody has dictates what treatment is available to them. If you are living with genotype 3, then there is evidence that liver disease might progress more quickly.
The ability of the virus to mutate has resulted in the existence of different genetic variations of HCV. These are called genotypes. The different genotypes are often, but not exclusively, related to different parts of the world.
Genotypes 1, 2 and 3 have a worldwide distribution. Types 1a and 1b are the most common, accounting for about 60% of global infections. They predominate in Northern Europe and North America and in Southern and Eastern Europe and Japan. Genotype 2 is less frequently represented than type 1. Genotype 3 is endemic in south-east Asia. Genotype 4 is principally found in the Middle East, Egypt, and central Africa. Type 5 is almost exclusively found in South Africa. The most common genotypes found in the UK are 1 and 3.
It is still unclear whether or not the type of virus affects the progression of the disease. If it does it is not thought to present any real cause for concern. However, HCV genotype does influence response to treatment. If you are considering treatment it is very important to know which genotype you are actually infected with.
Also Check: Signs Of Hepatitis C In Adults
Read Also: Can You Get Hepatitis C From Drinking After Someone
What Should I Do If I Think I Have Been Exposed To Viral Hepatitis
- If you may have been exposed to hepatitis A or B, your doctor may recommend getting a vaccine to keep you from getting the infection.22,23
- The CDC recommends that people who are exposed to hepatitis C, such as a health care worker after an accidental needle stick, get tested for hepatitis C infection.18 New antiviral medicines for hepatitis C cure most of the people who take them. If you have health insurance, ask about your copay and coinsurance and which medicines are covered under your plan.
Also Check: How Do I Know If I Have Hepatitis B
Eligibility Of Studies For Inclusion
Bibliographic details and abstracts of all citations retrieved by database and grey literature searches were downloaded into EndNote version X7. Any duplicated citations were excluded before first-pass screening. Citations were screened for eligibility by a single reviewer, based on the inclusion and exclusion criteria provided in Table . Studies that did not meet the inclusion criteria were checked by a second reviewer, to reduce the possibility of excluding a relevant report. Subgroups of particular interest comprised HCV GT3 patients with cirrhosis, prior treatment failure, and HIV co-infection, although a greater range of patient types was included in the studies identified and from which data were extracted prior to analysis for the review .
Table 1 Specification of population, intervention, comparison and outcomes
You May Like: How Is Hepatitis B Transmitted
Contaminated Needles And Infected Blood
You can get hepatitis C from sharing contaminated needles, syringes and other injecting equipment during recreational drug use. Banknotes and straws used for snorting may also pass the virus on.
Being exposed to unsterilised tattoo and body piercing equipment can also pass hepatitis C on. Occasionally, you can get it from sharing a towel, razor blades or a toothbrush if there is infected blood on them.
Hepatitis C infection is also passed on in healthcare settings, from needle stick injuries or from medical and dental equipment that has not been properly sterilised. In countries where blood products are not routinely screened, you can also get hepatitis C by receiving a transfusion of unscreened blood and blood products.
You can prevent hepatitis C by:
- never sharing needles and syringes or other items that may be contaminated with infected blood
- only having tattoos, body piercings or acupuncture in a professional setting, where new, sterile needles are used
- following the standard infection control precautions, if youre working in a healthcare setting.
Treating Hepatitis C Genotype : The 2019 Update
Since mid 2015 when I first began working helping people to access affordable Hepatitis C treatment with generic Hep C drugs from India it became clear that Hep C genotype 3 was the most difficult of the 6 common genotypes of Hepatitis C to cure. I learned that not only is Genotype 3 the most difficult variety of Hep C to cure but it also causes the most damage to the liver.
One of the first people I helped with treating Hep C G3 was an English guy living on the Spanish Island of Majorca. He caught Hep C from surgery after a motorcycle accident in India, so he found it ironic that both his disease and the medication for its cure came from India.In those days the optimum treatment for Hepatitis Genotype 3 was 24 weeks of Sofosbuvir + Ribavirin. This was before generic Daclatasvir became available in India.A few months prior to when he contacted me he had been ripped off by a couple of Russians who promised to supply him with the Sofosbuvir + Ribavirin for 10,000 Euros. He handed over the money and never saw those two men, or the money, again.So he was a little reluctant to send money to me, a person he had never met, living thousands of miles away. But we did manage to work that out and I sent him his genotype 3 treatment from India.
Hepatitis C genotype 3 is very common in South East Asia and also in some East European countries such as Serbia.
Hep C Genotype 3: A Global Problem
Also Check: How To Test For Hepatitis C At Home
Data Extraction From Citations
Full texts of citations deemed relevant during title and abstract screening were retrieved for second-pass review. These were assessed for eligibility by a single reviewer, with excluded studies checked by a second reviewer. For all eligible studies, data relevant to study design, trial characteristics, patient characteristics, disease characteristics and treatment outcomes were extracted, as detailed in Table .
Table 2 Data extraction
Overlapping reports were identified based on matching study names and/or trial numbers, settings, and authors. Sample sizes, years of data collection, and other study characteristics were compared between each citation, to select the most complete and informative data available for extraction. Data were extracted by a single reviewer into Microsoft Excel tables, which were checked by a second reviewer. Reviewers completed data extraction for all fields and noted where data in the relevant field was not reported.
Medications Used To Treat Hepatitis C
The HCV Medications section on this website provides detailed information for each of the Food and Drug Administration -approved medications listed in the treatment recommendations, including links to the full prescribing information and to patient assistance programs. The DAAs exert their action at specific steps in the HCV life cycle. There are three major classes of DAA medications: nonstructural proteins 3/4A protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors the NS5B polymerase inhibitors include the nucleoside analogs and nonnucleoside analogs. Adherence with the treatment regimen is of paramount importance. Thus, individuals should receive detailed counseling regarding the importance of adherence prior to starting therapy, as well as intensive monitoring and follow-up during therapy.
Don’t Miss: How Do You Get Rid Of Hepatitis B
Challenges In Treating Genotype 3 Hepatitis C Virus
In the United States, an estimated 3 to 4 million people have chronic hepatitis C virus infection.1 Among the 6 known genotypes of HCV, genotype 1 is the most prevalent and accounts for three-quarters of cases.1 Between 13% and 15% of HCV cases are genotype 2 , and approximately 10% are genotype 3 .2,3 Globally, the distribution is different, with GT3 HCV accounting for 75% of HCV infections in South Asia, 25% in Western Europe, and 10% to 20% of all HCV infections in North America.4
Early clinical trials of interferon monotherapy or IFN plus ribavirin suggested that patients with GT2 and GT3 were easier to cure than individuals with GT1.5-7 However, these early trials of IFN-based regimens grouped patients with GT2 and GT3 together and reported pooled outcomes data.4 When clinical trials for the novel IFN-free direct-acting antiviral therapies assessed outcomes by genotype, the findings upended conventional wisdom and showed GT3 was actually more difficult to cure than the other genotypes.2,4
Treatment-Naive Patients Without Cirrhosis
For treatment-naive patients with GT3 who do not have cirrhosis, recently updated collaborative guidelines from the Infectious Diseases Society of America and the American Association for the Study of Liver Diseases recommend starting with an 8-week course of Mavyret or with a 12-week course of Epclusa .8 Mavyret and Epclusa are fixed-dose combination oral tablets.
Donât Miss: Can Hepatitis C Cause Liver Cancer
Successful Treatment Of Hepatitis C Genotype 3 Treatment Failure With Sofosbuvir/velpatasvir In Decompensated Cirrhosis Complicated By Renal Insufficiency
1UOC Malattie Infettive, ASP 8 Siracusa, Italy
2Gastroenterology Unit, Di.Bi.Mis, University of Palermo, Italy
3Department for the Treatment and the Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT , UPMC , Palermo, Italy Department of Surgery and Medical and Surgical Specialties, University of Catania, Catania, Italy
Don’t Miss: How Long Does Hepatitis B Last
Hcv/human Immunodeficiency Virus Coinfection
Coinfection with HIV and HCV-3 is relatively common. According to a meta-analysis of over 780 studies, the global prevalence of HCV/HIV coinfection was estimated to be about 6% . In the US and Western Europe, the prevalence of coinfection is approximately 10-30% . Coinfection with HCV-3 increases the risk of chronicity while producing a higher viral load and an inability to mount a CD4/CD8 mediated T-cell immune response . Coinfection has also been associated with an accelerated progression of liver fibrosis and a reduced SVR to interferon-based regimens . However, antiretroviral therapy has been shown to ease the progression of HCV-associated liver injury and fibrosis by reducing HIV-related inflammation and immune dysfunction, and limiting infectivity . Additionally, HCV treatment in coinfected patients has been associated with a reduction in the frequency of death, HIV progression, liver-related events, and reduced hazards of diabetes mellitus and possibly chronic renal failure . Since HIV accelerates the natural history of HCV and liver-related complications, it is recommended that all coinfected patients be treated for chronic HCV, except those whose life expectancy is less than a few years and will not be remediated by treatment .
Sofosbuvir Plus Pegylated Interferon/ribavirin
The combination of SOF plus PegIFN/RBV for 12 weeks is the only interferon based therapy recommended by the EASL and AASLD guidelines for the treatment of HCV genotype 3 infection .
In naive non-cirrhotic patients, SOF plus PegIFN/RBV for 12 weeks resulted in an overall SVR of 92-100% . However, efficacy data is scarce: few patients were included in clinical trials and only three studies evaluated the SVR rates in this population. The phase II study included 25 naive non-cirrhotic patients treated with SOF plus PegIFN/RBV for 12 weeks, reaching an overall SVR rate of 92%, but no SVR data according to specific genotype is available 70033-1.). Another phase II study included 17 patients treated with SOF plus PegIFN/RBV for either 12 or 8 weeks and the overall SVR rate was 100% in both arms . The Boson phase III study included 71 naive non-cirrhotic patients with HCV genotype 3 infection treated with SOF plus PegIFN/RBV for 12 weeks, achieving an overall SVR rate of 96% .
In non-cirrhotic patients, including naive and with previous failure to PegIFN/RBV, SOF plus PegIFN/RBV for 12 weeks resulted in high SVR rates . It must be noted that non-significant differences in SVR rates were observed among naive and treatment-experienced patients, but these data need to be cautiously analyzed, since only small cohorts were included in the studies.
Read Also: Is Hepatitis Ca Sexually Transmitted Disease
Don’t Miss: When Was Hepatitis B Discovered
Hepatitis C And Health
How can health-care personnel avoid exposure to HCV?
Avoiding occupational exposure to blood is the primary way to prevent transmission of bloodborne illnesses among health-care personnel. To promote blood safety in the workplace, health-care personnel should consult infectious-disease control guidance from the National Institute for Occupational Safety and Health and from CDC. Depending on the medical procedure involved, Standard Precautions may include the appropriate use of personal protective equipment .
What is the risk of acquiring hepatitis C after being accidentally exposed to HCV-contaminated blood or body fluids in the workplace?
Although sharps injuries have decreased in recent decades due to improved prevention measures, they continue to occur, placing health-care personnel at risk for several bloodborne pathogens like hepatitis C. A recent analysis of several studies revealed an overall 0.2% risk for infection among those exposed to HCV-antibody-positive blood through needlestick or sharps injuries . Updated guidelines for management and treatment of hepatitis Cexternal icon are available to provide guidance for health-care personnel who become infected via exposure to contaminated blood at the workplace.
Other than needlesticks, do other exposures place health-care personnel at risk for hepatitis C?
Should HCV-infected health-care personnel be restricted in their work?
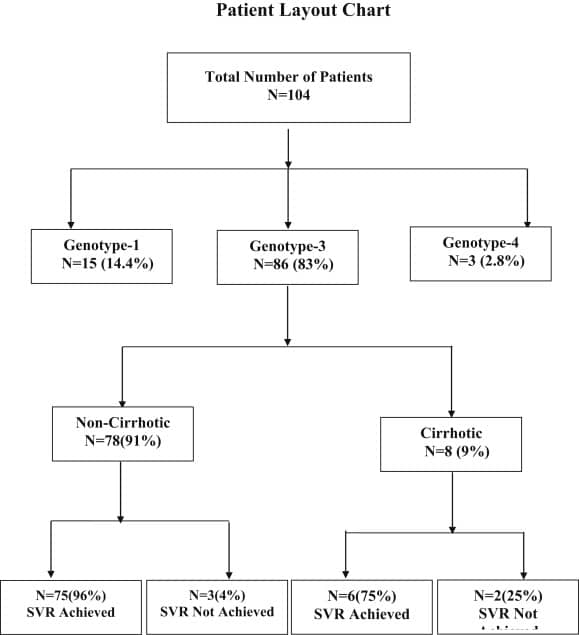
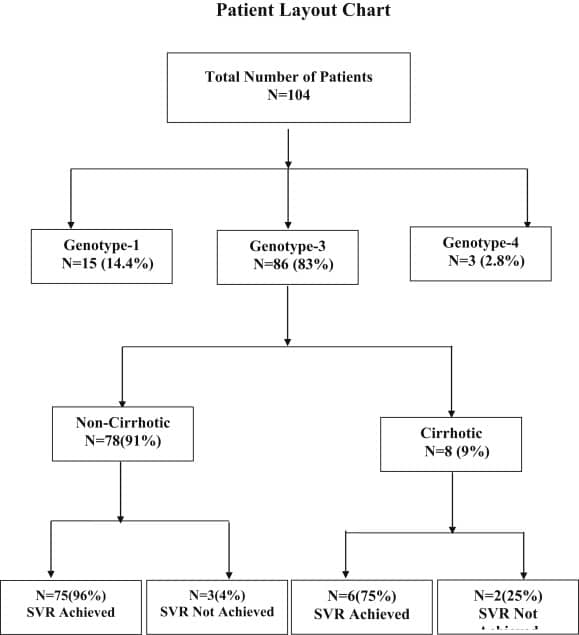
Efficacy And Safety Of Generic Daclatasvir + Sofosbuvir Ribavirin In Treatment Of Genotype 3 Infected Hepatitis C Patients
Muhammad Umar1 Tayyab Saeed Akhter1 Samar Saleem1 Shoaib Khokhar1
1Centre for Liver and Digestive Diseases, Holy Family Hospital, Rawalpindi Medical College and Allied Hospitals ,
Murree 47150, Pakistan .
Received:First Decision:Revised:Accepted:Science Editor:Copy Editor:Production Editor:
© The Author 2018. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Also Check: How Can You Get Infected With Hepatitis B