Seventeen Serological Patterns Of Hepatitis B
Seventeen serological patterns of hepatitis B were found out of 33,187 pediatric patients. Entire patterns and their distribution among these subjects were shown in Table 2. Four high-frequency patterns from pattern one to four were ‘anti-HBs alone’ , ‘negative pattern’ , ‘anti-HBc anti-HBs ‘ and ‘anti-HBe anti-HBc anti-HBs ‘ . The positive rates of other thirteen low-frequency patterns from pattern five to seventeen varied from 0.003% to 0.86% and no HBcAb-IgM was found in any of subjects. Except for ‘negative pattern’, ‘HBsAg HBeAg anti-HBc ‘, ‘HBsAg anti-HBe anti-HBc ‘ and ‘anti-HBe anti-HBs ‘, no significant differences were found between the distribution ratios and corresponding gender groups by chi-square test .
Demographic Characteristics And Risk Factors For Infection With Hepatitis B Virus
As presented in Table , we had more female and married participants aged 30 years and above .
Table 1 Summary of the sociodemographic characteristics of the study participants.
A total of 1273 reported for screening for HBsAg as a prerequisite for immunization against HBV over a period of 9 months at Kibuku Health center IV, of which 624 were below the age of consent and 182 turned down subsequent recruitment in the study. Forty-three participants were HBsAg seropositive representing a prevalence of 9.2% and 424 were HBsAg seronegative. There was 100% concordance between the two screening tests .
The HBsAb, HBeAb, HBcAb seropositivity was detected among 48, 73 and 45 participants respectively. In contrast, 327 of the participants did not present with any marker of past exposure to HBV as an infcetion or as a vaccine. .
Figure 2
Representative results of the one step hepatitis B virus combo test cassette for the HBV serological markers among the HBsAg negative participants.
Interestingly, 3, 26 and 3 were simultaneously positive for the two markers of HBsAb/HBeAb, HbeAb/HBcAb and HBsAb/HBcAb respectively. In addition, 12 were concomitantly positive for the three markers of HBsAb/HBeAb/HBcAb .
Table 2 Outline of the HBV serological profile of HBsAg seronegative participants screened from Kibuku Health center IV.Table 3 Socio-demographic factors associated with HBsAb serostatus among HBsAg seronegative hospital attendees screened for HBV before immunization.
Data Presentation And Statistical Analysis
Categorical data are presented as frequencies and percentages. Univariate analysis was used to determine the crude odds ratio , whereas multinomial logistic regression analysis was used to determine the adjusted odds ratio . All analyses were performed at the 95% level of significance, and p< 0.05 was considered to be statistically significant. Data were analyzed using SPSS version 26.
You May Like: What Happens If You Have Hepatitis
Study Area And Period
A hospital-based study was conducted between January to September 2020 at Kibuku Health Center IV in eastern Uganda. This study site was chosen purposively because it was a pilot site for HBV vaccination in the eastern region during the study period. Kibuku District has a population of 250,600 with an area of 489.1 km2 and a population density of 512.4/km2 at an elevation of 1100 m above sea level. Participants recruited in the study were from Kibuku District and the neighboring catchment areas.
Serological And Virological Profile Of Patients With Chronic Hepatitis B Infection In Eritrea
Mohammed Elfatih Hamida1*, Saud Mohammed Raja2, Yemane Seyoum2, Isam Mohammed Elkhidir3 and Freweini Tekle4
1Orotta College of Medicine and Health Sciences, Department of Microbiology, Asmara, Eritrea2Orotta College of Medicine and Health Sciences, Department of Internal Medicine, Asmara, Eritrea3University of Khartoum, Faculty of Medicine, Department of Microbiology, Khartoum, Sudan4Ministry of Health, National Health Laboratory, Asmara, Eritrea
*Address for Correspondence: Mohammed Elfatih, Orotta College of Medicine and Health Sciences, Department of Microbiology, Asmara, Eritrea, Email: mohelfatih77@gmail.com
Abstract
Background: Hepatitis B virus infection is a major cause of liver associated morbidity and mortality with diverse spectrum of disease. It is estimated about 15% to 40% of patients with hepatitis B virus infection progress to chronic hepatitis and about 15% to 25% die from disease complications. The main aim of this study was to evaluate the serological and virological markers of patients with chronic hepatitis B virus infection to determine the natural history of chronic hepatitis B infection in the Eritrean setting.
Background
Patients with HBV infection should be screened serologically to determine whether they have chronic infection or had suffered previous exposure to HBV. To that ends, it is necessary to test the serum for hepatitis B surface antigen , hepatitis B surface antibody , and antibody to hepatitis B core antigen .
You May Like: What Is The Meaning Of Hepatitis
Secular Trends In The United States
Hepatitis B became nationally notifiable as a distinct entity during the 1970s after serologic tests to differentiate different types of hepatitis became widely available.
In 2018, a total of 3,322 cases of acute hepatitis B were reported to CDC, for an overall incidence rate of 1.0 cases per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 21,600 acute hepatitis B cases occurred in 2018. The rate of reported acute HBV infections declined approximately 90% since recommendations for HepB vaccination were first issued, from 9.6 cases per 100,000 population in 1982 to 1.0 cases per 100,000 population in 2018.
During 2009 through 2013, the combined incidence of acute HBV infection in three states increased 114% and was associated with increasing injection-drug use. Incidence is greatest for persons age 40 through 49 years persons age 19 years or younger have the lowest incidence , likely a result of routine infant vaccination.
Although HBV infection is uncommon among adults in the general population , it is highly prevalent in certain groups. Generally, the highest risk for HBV infection is associated with lifestyles, occupations, or environments in which contact with blood from infected persons is frequent. Chronic HBV infection has been identified in 3.5% to 20.0% of persons who inject drugs in a variety of settings, and 22.6% of PWID have evidence of past infection.
Sample Size Determination And Sampling Procedure
The sample size was estimated by using the formula described by Cochran. A prevalence of HBV of 50% for no previously reported prevalence was used. Additionally, a standard normal deviation corresponding to the critical region of 1.96 at 5% precision was used. After correcting for the 10% loss due to unclear sample, a sample size of 394 was found to be sufficient. However, to raise the statistical power, an overall sample size of 424 participants was used. Purposive sampling was performed and any HBeAg sero-negative hospital attendee after screening was eligible for inclusion in the study.
Read Also: Rx Hepato Support For Dogs
Detection Of Hbv Serological Markers
A routine test panel consisting of five serological markers was employed to monitor HBV infection or vaccination efficacy for patients in our hospital. If HBsAg, HBeAg, or anti-HBc was positive for any subjects, anti-HBc would be tested further. Around 2 to 3 mL of venous blood for each subject was taken into vacuum-tube with coagulant, rested for at least half an hour at room temperature, then centrifuged for 3 minutes at 3,000 r/min to collect the sera. Five serological markers were detected on e601 analyzer according to Roche’s protocols by electrochemiluminescence immunoassay , where ‘sandwich principle’ for HBsAg, HBeAg, and anti-HBs, and ‘competition principle’ for anti-HBe and anti-HBc. The results for all samples reactive for HBsAg were confirmed by the confirmatory assays of Roche e601. All reagent kits, including calibrators and controls, were purchased from Roche Company and strictly used before the expiration date. Reference intervals for five serological markers were as follows: HBsAg < 1 COI , HBeAg < 1 COI, HBsAb < 10 mIU/mL, HBeAb > 1 COI, and HBcAb > 1 COI.
Similar Articles Being Viewed By Others
Carousel with three slides shown at a time. Use the Previous and Next buttons to navigate three slides at a time, or the slide dot buttons at the end to jump three slides at a time.
06 February 2019
Chikako Yamamoto, Ko Ko, Junko Tanaka
17 December 2020
Desalegn Admassu Ayana, A. Mulu, R. Howe
11 July 2022
Guo Yonghao, Chen Yanping, Guo Wanshen
21 April 2021
Masami Miyakawa, Lay-Myint Yoshida, Hiroyuki Moriuchi
18 May 2021
Jaya Singh Kshatri, Debdutta Bhattacharya, ICMR-RMRC Serosurvey Team
30 April 2020
Piyawat Komolmit, Vinita Oranrap, Yong Poovorawan
volume 12, Article number: 7425
Read Also: How To Treat Hepatitis C Naturally
Serological Diagnosis Of Hbv Infection
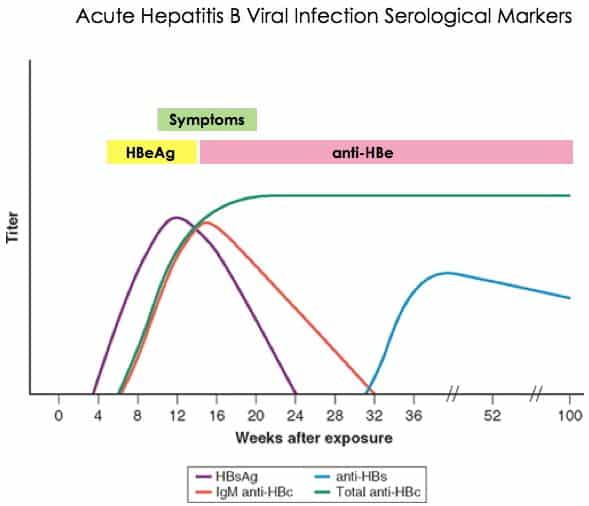
The definition and clinical use of common HBV serological markers are shown in . HBsAg is the hallmark of HBV infection and is the first serological marker to appear in acute hepatitis B. Most patients recovering from acute hepatitis B clear HBsAg within 4-6 months after onset of infection however, persistence of HBsAg for more than 6 months refers to chronic HBV infection. Anti-HBs is a neutralizing antibody and its presence suggests the recovery from hepatitis B and confers a long-term protective immunity against HBV infection. In addition, it is the only detectable serological marker in those who successfully respond to hepatitis B immunization.
Hepatitis B e antigen usually indicates active HBV replication and risk of transmission of infection. Seroconversion from HBeAg to anti-HBe is usually associated with serum HBV DNA undetectable by hybridization technology and remission of liver disease. Nevertheless, a certain proportion of anti-HBe-positive patients continue to have HBV replication and active liver disease. These patients usually harbor precore/core promoter mutations in the HBV genome that prevent or decrease the production of HBeAg. The prevalence and clinical significance of HBeAg-negative chronic hepatitis B have been increasingly recognized in many Asian countries.
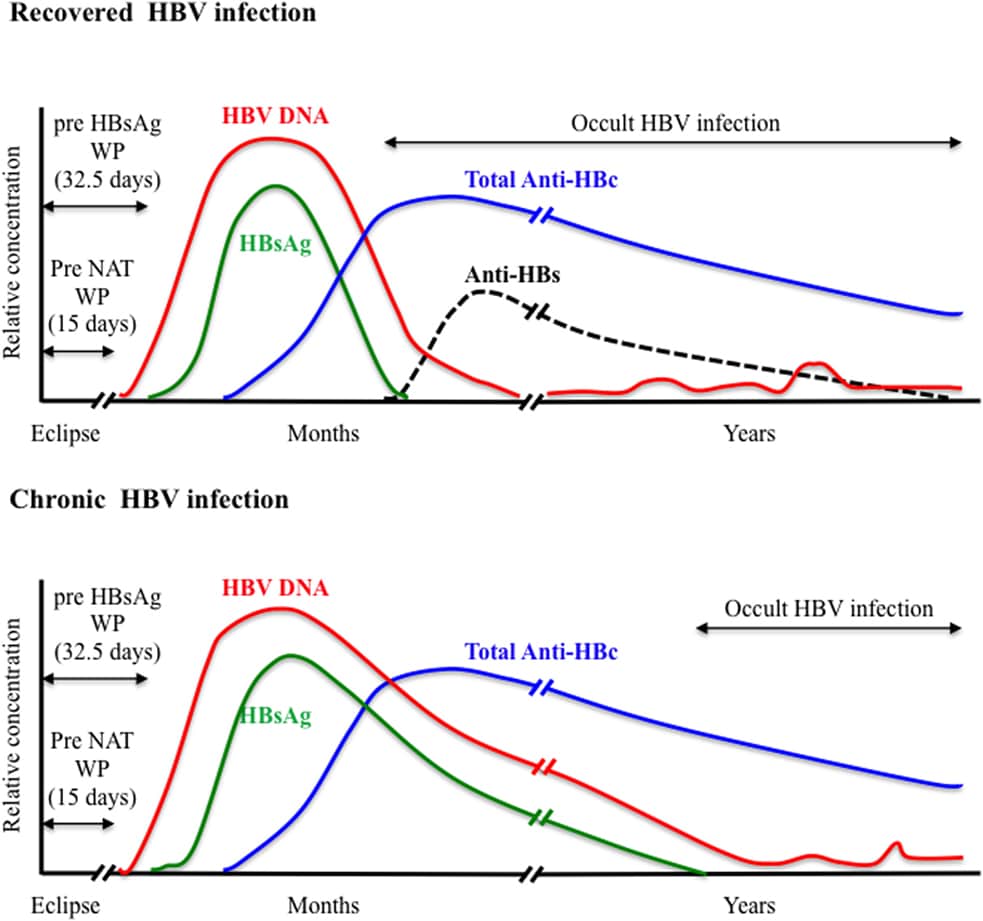
The typical serological profiles of acute HBV infection with recovery and progression to chronic HBV infection are shown in Figures 1A & 1B, respectively.
Figure 1.
Serological Markers For Hbv Infection
Serological markers for HBV infection consist of HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc IgM and IgG. The identification of serological markers allows: to identify patients with HBV infection to elucidate the natural course of chronic hepatitis B to assess the clinical phases of infection and to monitor antiviral therapy .
HBsAg is the serological hallmark of HBV infection. After an acute exposure to HBV, HBsAg appears in serum within 1 to 10 weeks. Persistence of this marker for more than 6 months implies chronic HBV infection . Several studies have reported the association between transcription activity of cccDNA in the liver and serum HBsAg levels . Differences in the serum HBsAg levels during the different phases of infection indicate the distribution of cccDNA during the respective phases of the disease. The serum HBsAg titers are higher in patients with HBeAg-positive CHB than in HBeAg-negative CHB . Monitoring of quantitative HBsAg levels predicts treatment response to interferon and disease progression in HBeAg-negative CHB patients with normal serum alanine aminotransferase levels .
Recommended Reading: Which Hepatitis Is Most Contagious
An Interesting Case Of Isolated False
Abstract
The standard serologic markers used to diagnose hepatitis B infection include hepatitis B surface antigen , hepatitis B surface antibody , total hepatitis B core antibody , and IgM antibody to hepatitis B core antigen . Different markers or combinations of markers are used to identify different phases of HBV infection and determine whether a patient has acute or chronic infection or immunity due to prior infection or vaccination or is seronegative and susceptible to future infection. Isolated HBsAg seropositivity is a peculiar serological pattern that requires investigation. Herein, we present a case of an asymptomatic female without a history of liver disease or evident risk factors for hepatitis, who underwent screening for infectious disease prior to resection of basal cell carcinoma involving her eyelid. The patients laboratory testing showed positivity for HBsAg and the HIV 1/2 screen. To investigate, we performed serial dilutions, utilized heterophilicantibody blocking tubes, and repeated analysis using a different commercial assay , all in support of a false-positive result attributed to a heterophilic antibody. Hence, we demonstrate that heterophilic antibody interference can result in isolated HBsAg positivity and recommend considering this form of interference in the differential where there is low clinical suspicion for viral infection.
1. Introduction
2. Case Report
3. Discussion
Data Availability
Conflicts of Interest
Molecular Methods For Hbv Infection
HBV DNA is a direct measurement of the viral load, which reveals the replication activity of the virus. It is detectable at the early stage of infection and increases up to peak level approximately 3 months after the exposure to HBV and then gradually diminishes in chronic infection or disappears at the recovery from HBV infection.
As the prevalence of serologically negative HBV infection has increased, HBV-DNA detection has obtained more awareness in clinical medicine . The detection of HBV DNA is a reliable marker of replication activity, and higher titers of HBV DNA are related to the more rapid disease progression and higher incidence of HCC . Furthermore, HBV DNA testing is useful in routine clinical setting to determine patients who need antiviral therapy and monitor them for suitable treatment .
There are two principles of techniques to identify and quantify HBV DNA: signal amplification such as hybrid capture and branched DNA technology target amplification such as polymerase chain reaction . Real-time PCR can detect wide dynamic range of viral load . For this reason, it has come to be the standard method to detect and quantify HBV DNA in clinical setting. Furthermore, it can be fully automated and does not generate carry-over contamination . Table 1 displays the comparison of assays for quantitative measurement of HBV DNA.
Read Also: Hepatitis B Symptoms In Men
Data Collection And Blood Sampling
Demographic characteristics and predictors of HBV infection were collected by using a close-ended questionnaire administered by a nurse. In addition to the demographic characteristics of age, sex and marital status, the participants were asked whether they had ever transfused blood, consumed alcohol or had lived with HBV-infected persons before. The questionnaire was administered by a nurse or research assistant on site. For laboratory investigations, 4 mL of blood was drawn by vein puncture into anticoagulant vacutainers from which serum was obtained. The serum was kept in sterile viols and stored at 20 °C until further use.
Hepatitis B Prevention Strategies
HepB vaccination is the mainstay of hepatitis B prevention efforts. A comprehensive strategy to eliminate HBV transmission includes universal vaccination of infants beginning at birth, routine vaccination of previously unvaccinated children less than age 19 years, and vaccination of adults at risk for HBV infection, including those requesting protection from HBV without acknowledgement of a specific risk factor. It also includes universal testing of pregnant women for HBsAg to identify newborns who require immunoprophylaxis for prevention of perinatal infection and to pregnant women who can benefit from antiviral therapy to reduce perinatal transmission.
Recommended Reading: Hepatitis C Signs And Symptoms Wikipedia
Hepatitis B Serologic Testing Methods
Luke Dang, MD, Scott Bainbridge, CLS, Nam Tran, PhD
Introduction
Hepatitis B virus was first discovered in 1965. Briefly, HBV is a DNA virus from the Hepadnaviridae family which is spread via contaminated body fluids. After exposure, the virus enters hepatocytes and integrates its circular, partially double-stranded DNA genome to the host cell nucleus as a covalently closed circular DNA intermediate, which acts as a stable nuclear template for viral replication. This mechanism enables the virus to chronically infect the host and reactivate at a later date ). Reverse transcription of the cccDNA then results in assembly and exocytosis of new viral particles. Hepatitis B exhibits geographic variation and is classified into 9 genotypes , with some differences in disease manifestations, although this remains an area of ongoing research.
Clinical Manifestations and Epidemiology
Diagnostic Serologic Testing for Hepatitis B
Hepatitis B screening should be performed on patients with signs and symptoms suggestive of acute or chronic hepatitis as well as asymptomatic individuals with a history of or high risk of exposure or those at high risk of complications .
Table 1. Interpretation of Hepatitis B Serological Results. Adapted from UpToDate. Hepatitis B virus: Screening and diagnosis. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018 67:1560.
Hepatology
References
Diagnosis Of Hepatitis B Infection
Acute hepatitis B is a clinical diagnosis identified by the detection of HBsAg, symptoms, high serum aminotransferases. Usually anti-HBc IgM can be detected and HBV DNA is present. HBeAg can also be identified in most acute phase of infections, but has little clinical importance. The diagnosis of chronic infection is based on the persistence of HBsAg for more than 6 months. Patients with chronic HBV infection are commonly diagnosed by laboratory means but not by clinical presentations. Past HBV infection is defined by the coexistence of anti-HBs and IgG anti-HBc.
Occult HBV infection is defined by persistence of low level of intrahepatic HBV DNA without detectable HBsAg . It is a serological situation defined by the presence of isolated anti-HBc with the absence of HBsAg and anti-HBs antibody . The detection of HBV DNA in the liver is the gold standard of diagnosis for occult HBV infection, since cccDNA remains in the hepatocytes and HBV DNA is occasionally identified in the liver but not in the serum. However, gaining hepatic HBV DNA is difficult in clinical setting since the procedure is invasive. Real-time PCR for serum HBV DNA detection have been shown with adequate sensitivity to identify occult HBV infection in many cases thus, HBV DNA testing is widely used to diagnose occult HBV infection .
Don’t Miss: Hepatitis B Vaccination How Long Does It Last
Hepatitis B Virus Dna
In patients with serological markers indicating infection, quantification of HBV-DNA is often performed.1 A high HBV-DNA viral load is associated with an increased risk of progression to cirrhosis and hepatocellular carcinoma .1 In patients with active chronic infection, a higher HBV-DNA viral load is expected.
Serologic Testing Of Vaccine Recipients
Prevaccination Serologic Testing
Vaccination of persons immune to HBV because of current or previous infection or HepB vaccination does not increase the risk for adverse events. However, in populations that have high rates of previous HBV infection, prevaccination testing might reduce costs by avoiding vaccination of persons who are already immune. Prevaccination testing consists of testing for HBsAg, anti-HBs, and anti-HBc. Serologic testing should not be a barrier to vaccination of susceptible persons, especially in populations that are difficult to access. Testing is not a requirement for vaccination, and in settings where testing is not feasible, vaccination of recommended persons should continue. The first dose of HepB vaccine should typically be administered immediately after collection of the blood for serologic testing. Prevaccination testing is recommended for household, sexual, or needle-sharing contacts of HBsAg-positive persons HIV-positive persons persons with elevated ALT/ AST of unknown etiology hemodialysis patients MSM and past or current PWID.
Serologic testing is not recommended before routine vaccination of infants, children, or adolescents.
Postvaccination Serologic Testing
Vaccine Nonresponse
- Persons who do not respond to the first HepB series should complete a second series on a 0, 1, 6 month schedule
- Retest anti-HBs 12 months after completion of second series
Vaccine Nonresponse
Recommended Reading: How To Convert Hepatitis B Positive To Negative