Hepatitis C Virus Epidemiology
The World Health Organization estimates that there are 130 to 150 million cases of HCV infection worldwide, totaling 3% of the worlds population, with an average of 3 to 4 million new infections occurring each year. HCV infection causes substantial morbidity, ranging from cirrhosis to hepatocellular carcinoma , liver failure, and death. HCV infection and HCC were the leading indications for liver transplantation in the United States in 2014 however, the spectrum for transplantation is expected to shift as more patients with HCV infection are identified and successfully treated. According to estimates in 2013, 3.2 million Americans have chronic HCV infection, yet only 50% of patients infected with HCV know of their viral status of those, 7% to 11% are treated, and 5% to 6% have successful clearance of the virus. Furthermore, it is predicted that the burden of cirrhosis due to HCV infection could reach 37.2% by 2020 in infected patients.
In order to identify patients infected with HCV, recommendations have been made for routine testing in people born between 1945 and 1965 in people who have injected illegal drugs, received blood transfusion or organ transplantation prior to July 1992, or received clotting factor concentrates before 1987 in patients on long-term dialysis in children born to HCV-positive mothers in health care workers who have been exposed to HCV infection and in patients with evidence of chronic liver disease.
What Is Hepatitis C
Hepatitis C, or hep C, is an infection of the liver caused by the hepatitis C virus . The virus is transmitted through the blood, most commonly through contaminated needles, but also through sex. In 75% to 85% of cases, the infection becomes chronic, which means the body cannot get rid of it. Chronic hepatitis C can slowly destroy the liver over several decades by causing liver cirrhosis and liver failure. It can also cause a type of liver cancer called hepatocellular carcinoma.
In the US, Latin America and Europe, the most common type of hepatitis C infection is type 1.
Here, well talk about treatment options for chronic HCV type 1, which makes up 60% to 75% of US cases of hepatitis C.
What About Patients With Hepatitis C Who Also Have Hepatitis B
Hepatitis B virus can flare in patients who are co-infected with hepatitis B and hepatitis C and are taking medication for hepatitis C. This has been reported as a potential risk for patients who are taking hepatitis C treatment and have underlying hepatitis B as well. The flare usually occurs within a few weeks after the patient starts taking medication for hepatitis C. Therefore, patients who have both hepatitis B and hepatitis C should be seen by a hepatitis expertbeforestarting treatment of the hepatitis C they may need to start taking hepatitis B treatment to avoid a hepatitis B flare.
Don’t Miss: Is Hepatitis C And Hiv The Same
Is Hep C Curable
The latest drugs available for hepatitis C have high success rates when it comes to curing the condition.
In conversations with your doctor, you can discuss the full range of treatment options. Some of these are combination drugs.
But its important to note that not every medication may be effective for you, even if its for the right genotype.
What Are The New Hep C Treatments
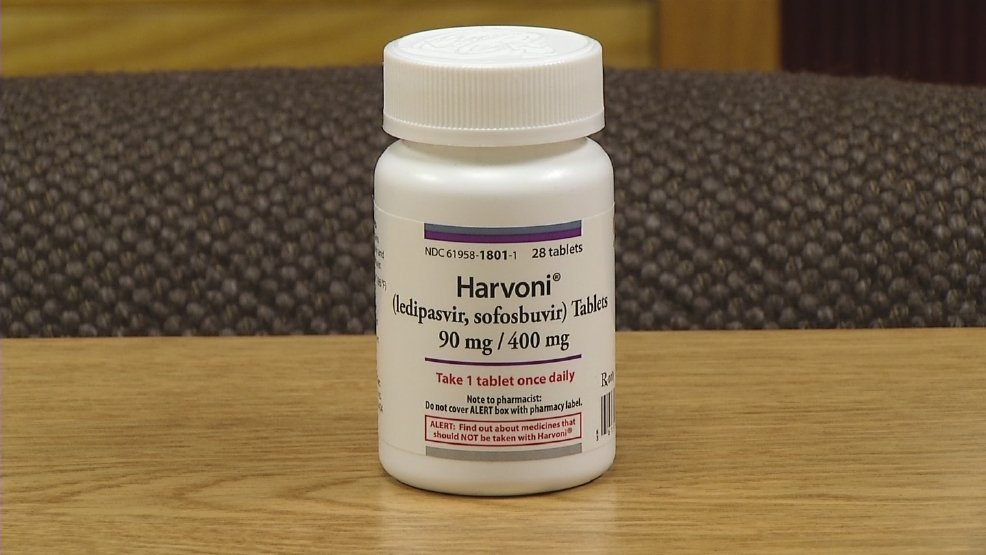
The new treatments for hep C type 1 include drugs like:
In the past, treatments for hepatitis C included interferon, a medication with many unpleasant risks and side effects. The cure rates were not that great either.
The new treatments are all interferon-free, so they have fewer side effects and risks than drugs containing interferon. Here are other benefits of Harvoni, Epclusa, and Mavyret:
-
Treatment courses are only 8 to 12 weeks long
-
Medication can be taken by mouth
-
Dosing requires only one tablet once a day
-
Cure rates are high
There are some otherequally effectivetreatment options, but these need more complex testing upfront and you will probably need to take more than 1 tablet per day:
*You may need to add with these treatments, depending on the subtype of HCV type 1 infection you have and whether or not you have liver cirrhosis.
Don’t Miss: How To Get Checked For Hepatitis
How Much Do The New Hep C Treatments Cost
The new hep C drugs are expensive. So expensive, that two of them make it onto our list of the 11 Most Expensive Drugs in the U.S.A. This is because they are still patented .
Luckily, hep C treatment is covered by most insurance plans, so for many people, the cheapest way of getting it will be through insurance .
If your hep C treatment is not covered by your insurance, ask your doctor about an appeal. The exact process will depend on your insurance provider but often requires that you work with your doctor to submit an appeal letter.
Other savings tips worth checking out:
Manufacturers $5 copay cards can make treatment more affordable :
A Researcher Reflects On Progress Fighting Hepatitis C And A Path Forward
The hepatitis C virus was discovered in 1989 research thats now earned a Nobel Prize.
When I began my medical career in Hong Kong in the early 1980s, I chose to focus on hepatitis B, in part because it was very common and because the hepatitis C virus had not yet been discovered. I witnessed the devastation that this virus caused cirrhosis, liver failure and liver cancer and the lack of treatments we could offer to patients.
Back then, scientists knew there was another type of hepatitis, but no one could identify it, so we called it non-A, non-B hepatitis. I would never have imagined that during the course of my career I would witness the discovery of what came to be known as hep C and the development of a cure for nearly all patients with chronic hepatitis C in 2014.
Underscoring the importance of these discoveries for global human health, this years Nobel Prize in Physiology or Medicine was awarded jointly to Harvey J. Alter, Michael Houghton and Charles M. Rice for the discovery of the hepatitis C virus.
Effective treatment for hepatitis C has become even more relevant today in light of the recent surge in new cases of hepatitis C due to rising opioid use.
You May Like: Hepatitis B Surface Antigen Test
Approach To Choosing Hcv Genotype 3 Treatment Regimen
When considering treatment of persons with chronic HCV genotype 3, five major factors influence the choice and duration of therapy: cirrhosis status, prior treatment experience, coexistent renal disease, drug interactions, and medication cost and/or insurance considerations. With certain regimens for treatment-experienced and/or cirrhotic patients, pretreatment NS5A resistance may also influence both the choice of regimen and duration of therapy. The following treatment recommendations are based on the 2013 AASLD/AST Evaluation for Liver Transplantation Guidelines for initial treatment of adults with HCV genotype 3 and for retreatment of adults in whom prior therapy failed, including those with HCV genotype 3.
Will The Drugs Be Available By 1 March 2016
Although the drugs will be available for prescribing through the Pharmaceutical Benefits Scheme, it may be that not all GPs or pharmacists are fully aware of the new treatments by that date. This means there may be some delay in some areas in accessing the drugs from your local GP. However, the Victorian Government is working with doctors, services and hospitals to ensure these delays are minimised as much as possible.
Recommended Reading: How Long Can You Live With Hepatitis C
Antiviral Treatment For Hepatitis C Reduces Risk Of Post
OSAKA, Japan – Hepatitis C virus -related hepatocellular carcinoma is a disease with a high recurrence rate.
Researchers at the Osaka City University Graduate School of Medicine reported in a new cohort study that in patients of HCC after receiving cancer treatment, the oral administration of direct-acting antivirals reduces the risk of tumor progression following recurrence of the liver disease.
The findings were published in the Journal of Viral Hepatitis.
Led by Norifumi Kawada, professor of the Department of Hepatology, the study investigated the effect eliminating HCV had on tumor progression* of early-stage HCC. “DAA therapy is effective at eradicating the hepatitis C virus, a major risk factor for HCC” says Professor Kawada. “While it is deemed low or inconclusive whether DAA therapy helps prevent HCC recurrence, little is known about how the antiviral therapy affects progression of the liver disease after cancer treatment.”
“Usually, cancer cells grow over long periods of time before they can be detected as a tumor,” states first author Hiroko Ikenaga. “Our study showed that eliminating the hepatitis C virus with DAA suppresses tumor progression, which we suggest contributes to overall patient survival.”
*Tumor progression was defined as when HCC progresses to more than 4 nodules in the liver, portal invasion or extrahepatic metastasis.
https://onlinelibrary.wiley.com/doi/10.1111/jvh.13627
Who Can Prescribe The New Drugs
A section 85 listing on the Pharmaceutical Benefits Scheme will allow general practitioners, as well as specialists, to prescribe the new treatments. This means that people with hepatitis C will be able to be treated by a general practitioner in the community. However, people with more advanced care needs, such as cirrhosis, may still need to see a specialist.
Also Check: How Long Can You Live With Hepatitis C Untreated
Nonstructural 3/4a Protease Inhibitors
The RNA genome of hepatitis C virus is translated into proteins intracellularly to carry out viral function. A NS3/4A serine protease cleaves unprocessed polyproteins to create functional, individual proteins, a process blocked by protease inhibitors. The NS5A protein helps with HCV RNA replication regulation and viral assembly and packaging, and directly interacts with the RNA-dependent RNA polymerase . NS5A inhibitors prevent hyperphosphorylation of the NS5A protein and alter the proteins location from the endoplasmic reticulum. NS5B polymerase inhibitors have a high barrier to resistance and work broadly against genotypes with intermediate potency. Nucleoside inhibitors arrest RNA synthesis, while nonnucleoside inhibitors bind and disrupt the RdRp function.
Are New Drugs For Hepatitis C Safe A Report Raises Concerns
- Read in app
By Denise Grady
Drugs approved in recent years that can cure hepatitis C may have severe side effects, including liver failure, a new report suggests.
The number of adverse events appears relatively small, and the findings are not conclusive. But experts said the report was a warning that should not be ignored. It involves nine widely used antiviral drugs that were heralded as a huge advance because they greatly increased cure rates, seemingly with few side effects.
The report will be published online on Wednesday by the Institute for Safe Medication Practices, a nonprofit in Horsham, Pa., that studies drug safety. Its findings are based on the groups analysis of the Food and Drug Administrations database of reports from doctors around the world of adverse events that might be related to medications.
Dr. Robert S. Brown, the director of the Center for Liver Disease and Transplantation at NewYork-Presbyterian at Columbia and Weill Cornell, who was not involved in the study, said that there had been other, scattered accounts of problems with the new drugs and that they should be investigated further.
We dont want people to ignore it and lead to risks to patients, he said. We dont want people to overreact and not treat patients who should be treated. A lot of doctors are unclear about it, and if doctors are unclear, patients are, too.
There is no vaccine to prevent the infection, so treatments have long been eagerly sought.
Also Check: Hepatitis C Is It Curable
What Happens If Hep C Meds Dont Work
In rare cases , the body wont respond to either medication. If this happens, your doctor may prescribe a more potent combination of antivirals such as those found in Vosevi.
This medication blends three anti-viral meds: sofosbuvir, velpatasvir, and voxilaprevir. Because its more powerful, it may have a higher burden of side effects like headache, fatigue, diarrhea, and nausea.
Dosage entails one pill taken once a day with food for 12 weeks. Your doctor may also keep you on the meds longer to clear the infection.
How Effective Is Hepatitis C Medication
Todays treatments are incredibly effective. According to the FDA, they have a 90-to-100% cure rate in just two to three months. A few months after your prescribed course of treatment is finished, your doctor will order a blood test to measure how much viral genetic material is in your blood. If none is visible, then youre considered cured. Note: These medications arent vaccines. There is no vaccine for hep C. While researchers continue to work on a vaccine in order to reach the World Health Organization’s goal of reducing infections by 80% in 2030, they have yet to be successful. So if you engage in risky behaviors, the condition could come back. It’s in your hands to live your life fully, safely!
Don’t Miss: Is There A Vaccine Available For Hepatitis B
First Hepatitis C Treatment Developed Through South
- New treatment combination for hepatitis C virus is an additional affordable option for millions still waiting for access to lifesaving treatments in middle-income countries
- Combination is safe and effective, including for hard-to-treat cases and people with HCV and HIV
- New drug ravidasvir is the first HCV drug to be developed through South-South collaboration and with support from non-profit organizations
The National Pharmaceutical Regulatory Agency of Malaysia has granted a conditional registration for a safe, effective hepatitis C treatment developed by a public-private partnership bringing together the Malaysian Ministry of Health, not-for-profit research and development organization Drugs for Neglected Diseases initiative , Egyptian pharmaceutical company Pharco, Malaysian pharmaceutical company Pharmaniaga Berhad, and non-governmental-organization Médecins Sans Frontières/Doctors Without Borders .
This is the very first drug for HCV to be developed through South-South collaboration and with funding and clinical support from non-profit organizations.
The approval on 4 June concerns a new drug, ravidasvir, for the treatment of chronic HCV infection in adults in combination with other medicinal products. Ravidasvir was developed by the partnership for use with sofosbuvir, an existing DAA, as an affordable, simple, and efficacious public health tool.
Plans to register the new ravidasvir + sofosbuvir combination are advancing in other countries, including in Latin America.
A Pricey Drug And New Generics
The first combo pill with two drugs that inhibits different steps in hepatitis C replication was approved by the FDA in 2014. This pill is taken once a day for 8-12 weeks, has little to no side effects and improved the cure rate to 90-95%. It was hailed as a magical cure, but it came with a price tag of US$94,500 for a 12-week course of treatment. That led many insurers in the United States and national health departments in other countries to limit access to treatment.
Since then, several othercombo pills withsimilar cure rates that are equally well-tolerated have become available, and the cost has markedly decreased. In addition, low-cost generics and special pricing arrangements are available in many resource-limited countries.
While the current price of hepatitis C virus drugs is still very high, one needs to remember that for 95 percent of patients, this is a cure. It is unlike medicines for many illnesses that need to be taken for a long time, sometimes for the rest of the patients lives. Indeed, a cure for hepatitis C virus has allowed some patients who were on the liver transplant waiting list to reverse their liver failure, making transplantation unnecessary. This is good news not only for these patients but also for others on the waiting list.
Recommended Reading: Help With Hepatitis C Treatment
Factors To Consider Prior To Choosing Retreatment Regimen
For retreatment of adults with HCV genotype 3 infection, several factors influence the regimen choice, including the prior regimen used when treatment failure occurred, the presence or absence of cirrhosis, and cost or insurance considerations. It is also worth noting that the clinical data for treatment-experienced individuals with HCV genotype 3 is more limited for the newest DAAs, such as glecaprevir-pibrentasvir, since these individuals have been encountered less frequently in recent years due to the efficacy of earlier DAA regimens. Therefore, the optimal duration of therapy for retreatment of persons with HCV genotype 3 with glecaprevir-pibrentasvir is not well established. The retreatment of individuals with HCV genotype 3 who have decompensated cirrhosis, renal impairment, acute HCV, or post-liver transplantation is not addressed in this lesson.
Can Hepatitis C Be Treated
Yes, since 2010 enormous progress has been made in the treatment of chronic hepatitis C. New therapies called direct-acting antivirals are pills that act on the virus itself to eradicate it from the body, unlike older medicines like interferon injections which work by stimulating an immune response. These new treatments are very effective and can achieve cure rates of over 90%. In most situations now, there is no need for interferon, which was responsible for many of the side effects previously associated with HCV treatment. The new treatment combinations require shorter treatment durations , have reduced side effects and appear to be effective at all stages of the disease.
Because these new therapies are very new, they remain very expensive. As such, drug coverage from both government and private companies may require that your liver disease has progressed to a certain stage before they are willing to cover the cost of these drugs.
Your primary care physician may refer you to a specialist to determine whether you are eligible for treatment. A specialist will help you decide which drug therapy is best for you based on the severity of your liver disease, your virus genotype and whether or not you have been treated in the past.
Recommended Reading: Hepatitis B Vaccine For Infants Schedule
Side Effects Of Treatment
Treatments with direct acting antivirals have very few side effects. Most people find DAA tablets very easy to take.
You may feel a little sick and have trouble sleeping to begin with, but this should soon settle down.
Your nurse or doctor should be able to suggest things to help ease any discomfort.
You need to complete the full course of treatment to ensure you clear the hepatitis C virus from your body.
If you have any problems with your medicines, speak to your doctor or nurse straight away.
Side effects for each type of treatment can vary from person to person.
For a very small number of people, more severe side effects from hepatitis C treatments may include: