Interfering With Viral Replication
Viral targets
In contrast to other RNA viruses that encode RNA-dependent RNA-polymerases for replication and mRNA synthesis, HDV recruits and reprogrammes the cellular Pol II to achieve these goals. Accordingly, an important viral drug target is lacking. Nevertheless, crucial steps in the viral life cycle like the ribozyme-mediated self-cleavage of genomic and antigenomic RNA oligomers or the HDAg-dependent regulation of RNA replication and mRNA synthesis are attractive viral structures suitable for drug targeting. Inactivation of the S-HDAg could induce a selective shut down of RNA synthesis . Alternatively, abolition of the interaction of the prenylated C-terminus of L-HDAg with the cytosolic loop in the HBV S-domain by small molecules would inhibit virus release similar to LNF, which targets the corresponding host enzyme . No such drugs have been identified so far, however applying the new replication systems mentioned above will facilitate screening approaches and drug candidate identification in the future.
Cellular targets
Beside these well-characterised host factors additional approaches using siRNA or drug libraries in susceptible cell lines will allow to identify novel host factors in the future and it will be a challenging task to identify those that allow intervention and are tolerable regarding side effects.
Assembly And Infection Efficacy Of Hepatitis B Virus Surface Protein Exchanges In 8 Hepatitis D Virus Genotype Isolates
- Wenshi WangCorrespondenceCorresponding author. Address: Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, Im Neuenheimer Feld 344, D-69120, Heidelberg, Germany. Tel.: +49 6221 564902 fax: +49 6221 561946.
- Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, Heidelberg, GermanyGerman Centre for Infection Research , partner site Heidelberg, Heidelberg, Germany
- Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, Heidelberg, GermanyGerman Centre for Infection Research , partner site Heidelberg, Heidelberg, Germany
- Stephan UrbanCorrespondenceCorresponding author. Address: Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, Im Neuenheimer Feld 344, D-69120, Heidelberg, Germany. Tel.: +49 6221 564902 fax: +49 6221 561946.Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, Heidelberg, GermanyGerman Centre for Infection Research , partner site Heidelberg, Heidelberg, Germany
- § Contributed equally
- HDV 1-8 presented marked differences in replication efficacy.
- All HDV/HBV combinations supported HDV assembly with varied kinetics.
- All HDVs elicited robust and comparable innate immune responses.
- EMA-approved entry inhibitor bulevirtide showed pan-genotypic efficacy against HBV-enveloped HDV.
- Lonafarnib, the assembly inhibitor showed pan-genotypic activity against HDV.
Similar Articles Being Viewed By Others
Carousel with three slides shown at a time. Use the Previous and Next buttons to navigate three slides at a time, or the slide dot buttons at the end to jump three slides at a time.
08 January 2021
Sarah Kadelka, Harel Dahari & Stanca M. Ciupe
20 October 2020
Ohad Etzion, Harel Dahari, Amir Shlomai
21 August 2019
Lorena Vigón, Sonia Vázquez-Morón, The GESIDA 3603b Cohort Study Group
volume 10, Article number: 7837
Also Check: Can Hepatitis C Go Away
Molecular Interactions Between Hbv And Hdv
HBV, with a 3.2 kb partial double stranded DNA, plays the role of a HDV helper virus in HBV/HDV-infected hepatocytes. From the four overlapping reading frames in the HBV genome one encodes viral surface envelope proteins. This region contains the pre-S1 , pre-S2 and S domains. These domains encode the large , middle and small HBV surface proteins which are all in the same translational frame with different start codons .
Although the S-HBsAg alone is sufficient for virion development due to its self-assembling trait , the presence of large HBsAg is necessary for both HBV and HDV to infect other cells. In-vivo studies have shown that all three HBsAg proteins are present in HDV particles. Delta proteins can bind to the S domain of HBV envelope as well as the L4 region which is located in the pre-S1 domain of HBsAg . This is the same region which HBV core proteins interact with.
What Is Hepatitis D
The hepatitis D virus is an RNA virus discovered in 1977 that is structurally unrelated to the hepatitis A, B or C virus. HDV causes a unique infection that requires the assistance of viral particles from hepatitis B virus to replicate and infect other hepatocytes. Its clinical course is varied and ranges from acute self-limited infection to acute fulminant liver failure. Chronic liver infection can lead to end-stage liver disease and associated complications. HDV infection occurs more commonly among adults than children. It is observed more commonly among patients with a history of intravenous drug use and in persons from the Mediterranean basin.
Recommended Reading: Homeopathic Medicine For Hepatitis C
Evolution And Diversity Of The Human Hepatitis D Virus Genome
Szecheng J. Lo
Abstract
Human hepatitis delta virus is the smallest RNA virus in genome. HDV genome is divided into a viroid-like sequence and a protein-coding sequence which could have originated from different resources and the HDV genome was eventually constituted through RNA recombination. The genome subsequently diversified through accumulation of mutations selected by interactions between the mutated RNA and proteins with host factors to successfully form the infectious virions. Therefore, we propose that the conservation of HDV nucleotide sequence is highly related with its functionality. Genome analysis of known HDV isolates shows that the C-terminal coding sequences of large delta antigen are the highest diversity than other regions of protein-coding sequences but they still retain biological functionality to interact with the heavy chain of clathrin can be selected and maintained. Since viruses interact with many host factors, including escaping the host immune response, how to design a program to predict RNA genome evolution is a great challenging work.
1. Introduction
However, no single hypothesis can satisfactorily explain the origin all known viruses. In this paper, we use the smallest human RNA virus, HDV, to illustrate how this virus could have evolved and diverged. The evolution of larger genome size of RNA viruses, such as retroviruses, flaviruses, picornavirus, and corona viruses, is not a subject in this review.
2. Molecular Biology of HDV
Etiologic And Clinical Manifestation
Hepatitis D virus is a small 36 nm single-stranded negative sense RNA virus that requires the presence of hepatitis B virus for its assembly and replication. Co-infection and super-infection represent two types of HDV known infections. During co-infection, the patient acquires HDV and HBV at the same time while super-infection occurs when a patient with chronic HBV infection becomes infected with HDV. Hepatitis D virion is composed of an outer lipoprotein envelope made of the surface antigen of the HBV and an inner ribonucleoprotein structure in which the HDV genome resides. HDV produces one protein with two forms a 27 kDa large-HDAg , and a small-HDAg of 24 kDa . The N-terminals of the two forms are identical they differ by an additional 19 amino acids in the C-terminal of the large HDAg. These two proteins play diverging roles during the course of an infection. HDAg-S is produced in the early stages of an infection and enters the nucleus supporting viral replication. HDAg-L, in contrast, is produced during the later stages of an infection, acts as an inhibitor of viral replication, and is required for the assembly of viral particles. Eight different genotypes of HDV have been identified, each with different geographic distribution and distinct clinical course. Genotype I shows wide geographic distribution including Europe and the United States.
Also Check: How Many Genotypes Of Hepatitis C Are There
Hdv And Hepatocellular Carcinoma
It has already been mentioned that cells infected with HDV appear to have altered gene expression and cellular responses, which is also evident from augmented expression of pro-inflammatory, growth and anti-apoptotic factors. It is thus explanatory that severe liver damage and a concomitant increased hepatic cell survival in HDV-infected patients may lead to HCC.
It is well known that nuclear factor kappa B dysregulation is associated with inflammation and cancer. L-HDAg has been shown to activate nicotinamide adenosine denucleotide hydro-phosphoric acid oxidase-4 which in turn induces oxidative stress. L-HDAg is therefore able to activate the signal transducer and activator of transcription-3 and the NF-B through the oxidative stress pathway. L-HDAg may also stimulate TNF- induced NF-B, probably via TNF receptor-associated factor 2 , a protein involved in the early signal transduction events. This may underscore a possible underlying cause of severe necroinflammation in HDV infection and its progression to HCC. A clinical study has also suggested that HCC in HDV infection may be a secondary effect of the necroinflammation and cirrhosis. In this study, decreased liver size was noticed more in cases of HDV HCC compared to HBV monoinfection group where the liver size was normal or increased. HDV patients had lower platelets and larger varices on endoscopy.
Immune Response To Hdv
Mechanisms of HDV-specific CD8+ Tcell failure. HDV-specific CD8+ T cells targeting viral epitopes with wild-type sequence display a chronically activated phenotype and are functionally partially exhausted , HDV-specific CD8+ T cells targeting viral epitopes with sequence variations do not recognise the antigen anymore and display a memory-like phenotype . HDV, hepatitis D virus.
Read Also: Letsgetchecked At Home Hepatitis B & C Test
Usmle Step 1 Style Questions Usmle
A 47-year-old man presents to the emergency department with altered mental status. He was found unconscious in an alley by a bystander who called paramedics to the scene. Upon arrival in the emergency department, the patient is mumbling but is unable to report his medical history. Chart review demonstrates a history of intravenous drug use and infection. Temperature is 36 °C , is 112/min, respirations are 10/min, is 100/53 mmHg, and oxygen saturation is 95% on room air. Physical examination demonstrates , track marks on the , as well as diffuse . The patient is appropriately , and the following laboratory results are seen:This patient’s clinical presentation is most consistent with infection with which of the following pathogens?
, or Hep B virus for short, is a member of the hepadnavirus family , or Hep D virus, is a deltavirus. They both cause , or inflammation of the liver. Even though they both cause , cannot cause the disease by itself, and needs to replicate.
Alright, now, both these viruses target the liver, which is made of functional units called . The main cells are called . They pick up and detoxify harmful substances like drugs or help maintain a normal synthesize a variety of important proteins, like albumin and store certain vitamins and some and convert into bile salts, which, along with water and , make up the bile.
Now, delta antigens are harmful to the cell and cause cell death and , so unlike hep B, hep D damages the cells directly.
Receptor Interaction And The Consequence Of Hbv Integration
Liver tropism and hepatic receptors of HBV and HDV
The liver tropism of HBV and HDV is primarily determined by a specific interaction of an extended receptor binding domain in the preS-1-part of the HBV L-protein and the hepatic NTCP receptor. NTCP interaction of HBV and HDV requires prior attachment to heparan sulphate proteoglycans . This mandatory step presumably triggers the release of the otherwise hidden preS-receptor binding site. HSPG-requirement explains how neutralising anti-HBsAg-specific antibodies, although they do not directly interfere with preS/NTCP-interaction, block entry and control infection.
NTCP exclusively locates at the basolateral/sinusoidal membrane of differentiated, polarised hepatocytes. NTCP-expression ceases when differentiated hepatocytes proliferate. NTCP is also downregulated in transformed cell lines of hepatic origin such as HepG2, HuH7 and Hep3B. Thus, proliferating normal hepatocytes, transformed hepatoma cells, and probably also tumour cells in HCC lack NTCP and do not support entry of HBV and HDV. Though, proliferating cells support spread of HDV RNA activated state) but loose HBV cccDNA . A deeper understanding of these peculiar differences of HDV and its helper HBV will be crucial for understanding persistence and is important for the development of successful therapeutic interventions.
Studying HDV replication in vitro
Consequences of HBV integration and clonal expansion of integrants
Don’t Miss: Symptoms Of Acute Hepatic Porphyria
Open Questions And Future Directions
-
Can hepatitis D virus establish transcriptionally silenced but reactivatable episomes in hepatocytes as an additional mechanism of persistence?
-
To what extent do the eight HDV genotypes differ in replication efficacy and sensitivity against the upcoming novel treatments?
-
Do HDV-targeted therapies lead to a restoration of HDV-specific immunity?
-
Are these restored immune responses required for treatment response? Are they required for prevention of viral relapse? Of note, there may be differences in treatment regimens that are associated with alanine aminotransferase flares combination therapy) compared with treatment regimens without ALT flares .
-
How can a synergistic potential of antiviral drugs and immune-modulators be translated into curative regimens?
-
Are there baseline or on-treatment predictors to sustained HDV virological response for the different treatment strategies?
-
Is a sustained HDV virological response without hepatitis B surface antigen loss a realistic and achievable aim for treatment regimens without IFNs?
-
Are drugs aiming at HBsAg loss effective and safe in patients with chronic hepatitis B virus/HDV coinfection?
-
Last but not least, since HDV is prevalent in low-income countries and migrant populations, it will be important to establish new concepts to foster diagnosis and access to care.
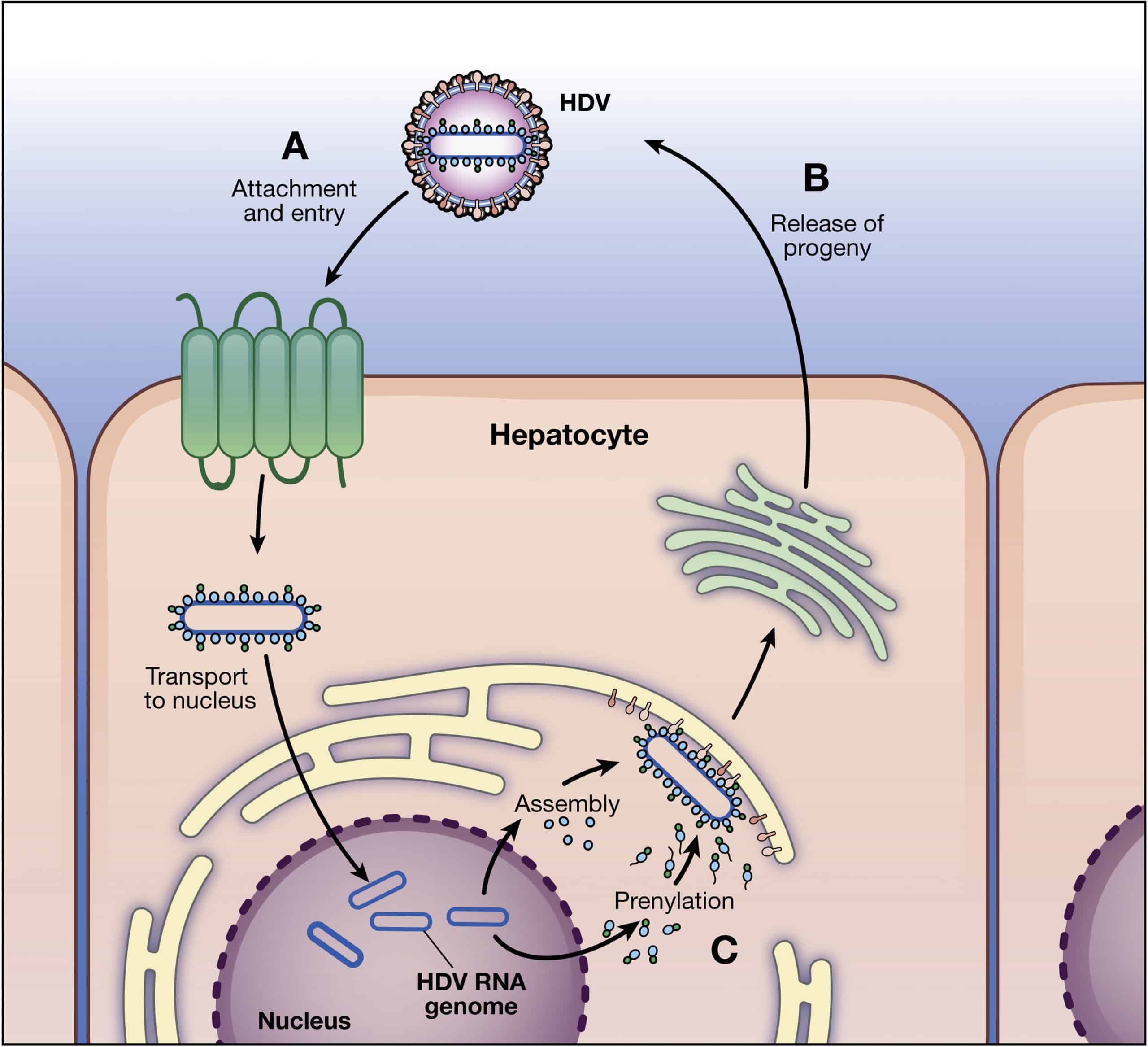
Cell Attachment Entry Uncoating And Replication
The mechanism of entry of the HDV into the hepatocytes is not clearly understood, however, it is thought to be similar to HBV. HDV enters hepatocytes by binding to the carbohydrate side chains of heparin sulphate proteoglycan present on the surface of hepatocytes. The N-terminal aminoacids of the pre-S1 domain of L-HBsAg are thus obligatory to HDV entry into hepatocytes. Mutations/deletions in highly conserved pre-S1 sequence and acetylation or myristoylation of pre-S1 N-terminal amino acids have been found to inhibit HDV entry into hepatocytes. Recently, Yan et al have identified a putative receptor for the entry of HBV and HDV into the hepatocytes. The authors proposed that pre-S1 domain of L-HBsAg interacts with sodium-taurocholate cotransporting polypeptide, an integral transmembrane glycoprotein involved in enterohepatic circulation, to facilitate HDV infection.
After HDV enters the cell, the uncoating of viral particle occurs and HDAg translocates the viral genome into the nucleus where RNA polymerasesIand II are employed to replicate the genome .1). Polymerase I involves the transcription of antigenome from viral genome in the nucleolus, while polymerase II catalyzes genome replication from antigenome and transcription of mRNA in the nucleoplasm.
Hepatitis D virus replication cycle. HBV: Hepatitis B virus HDV: Hepatitis D virus.
Read Also: Where To Get Hepatitis B Vaccine In Nigeria
High Serum Levels Of Hdv Rna Are Predictors Of Cirrhosis And Liver Cancer In Patients With Chronic Hepatitis Delta
-
* E-mail:
Affiliation AM & A Migliavacca Center for Liver Disease, 1st Division of Gastroenterology, IRCCS Fondazione Cà Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy
-
Affiliation Department of Transfusion Medicine and Hematology, Ospedale A. Manzoni, Lecco, Italy
-
Affiliation Dipartimento di Scienze Biomediche e Cliniche L. Sacco, University of Milan, Milan, Italy
-
Affiliation Department of Transfusion Medicine and Hematology, Ospedale A. Manzoni, Lecco, Italy
-
Affiliation AM & A Migliavacca Center for Liver Disease, 1st Division of Gastroenterology, IRCCS Fondazione Cà Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy
Life Cycle Of Hdv And Its Dependence On Hbv As Helper Virus
Hepatitis delta virus is a unique human pathogen, and has been the only known species in the genus Deltavirus , but was reclassified in a new family Kolmioviridae, genus Deltavirus within one new realm Ribozyviria . Due to the possession of a circular RNA genome and its mechanism of replication, similarities exist with viroids, which represent a large family of subviral plant pathogens . But HDV is clearly distinguished from the viroids by its larger genome size and the ability to encode a protein. The recent discovery of delta-like agents in various animal species has broadened the views on the evolutionary history of HDV .
The existence of HDV was discovered in 1977 by the identification of a new antigen, the delta antigen , in liver biopsies and sera from patients with a severe form of hepatitis B . Experimental transmission studies , then the cloning of the HDV genome demonstrated that the HDAg is associated with a separate transmissible agent. HDV is a satellite virus and depends on the human hepatitis B virus surface proteins for packaging, release, and transmission.
You May Like: Hepatitis C Treatment Cost 2020
Hdv Genotypes And Delta
Natural HDV infections have only been described in humans, and hence HDV most likely co-evolved with the helper HBV in the human lineage. Experimental transmission of HDV and HBV to chimpanzees , and the acceptance of mammalian hepatitis B viruses , such as the woodchuck hepatitis B virus as alternative helper viruses for HDV assembly and transmission allowed the establishment of animal models to study HDV replication and pathogenesis .
Eight distinct HDV genotypes have been documented in human populations. The HDV genotypes differ in their genomic sequence by 19â40% , and can be further sub-categorized into two to four subgenotypes with the exception of HDV genotype 3 . Their global distribution is geographically distinct except for HDV-1d . The subgenotype HDV-1d is prevalent worldwide, and represents the dominant HDV strain in Europe and North America. In contrast, HDV-1a and -1b are predominantely found in Africa and the Middle East, while HDV-1c is the dominant strain in the Western Pacific region . The subgenotypes HDV-2a, HDV-4a, and HDV-4b are mainly distributed in Southeast Asia, China, Japan, and Taiwan HDV-2b in Russia . HDV-3 is mainly located in the Northern part of South America, and HDV-5 to HDV-8 are found in Africa .
Hepatitis Delta Virus And Delta
- 1Victorian Infectious Diseases Reference Laboratory , Melbourne Health, The Peter Doherty Institute, Melbourne, VIC, Australia
- 2School of Science, Royal Melbourne Institute of Technology University, Melbourne, VIC, Australia
- 3The Peter Doherty Institute, University of Melbourne, Melbourne, VIC, Australia
Don’t Miss: What Part Of The Body Does Hepatitis C Affect
Protein Modifications Of Hdv
The level of HDV replication and pathogenicity is not only influenced by interactions with HBV and HBV mutants, but might be also impacted by modifications of the HDV proteins as well. As such, some amino acid residues in S- and L-HDAg appear to be critical for posttranslational modifications. Of these residues Arg-13, Lys-72 and Ser-177 in S-HDAg undergo methylation, acetylation and phosphorylation, respectively.
There are also multiple lysin residues throughout the whole S-HDAg as well as 66 amino acids at the N-terminal part of this protein which act as sumoylation sites of the protein. Sumoylation is a reversible process which has implications for cell cycle progression, nuclear import, regulation of transcription, protein turnover and other cell biology functions. In case of HDV, sumoylation enhances G-RNA and mRNA synthesis by unknown mechanism but has no effect on antigenomic RNA synthesis.
In L-HDAg Cys-211 gets isoprenylated for virus assembly. Deletion of 15 amino acids upstream of the isoprenylation site would also lead to the eradication of viral replication. Moreover, some mutants of HDV have been observed which can only replicate in the presence of wild-type HDV, called defective viruses.