Chemistry Mechanism Of Action Spectrum And Resistance
Simeprevir is an inhibitor of the NS3/4A serine protease of HCV, which is necessary for the proteolytic cleavage of the HCV-encoded polyprotein and is essential for viral replication. Simeprevir has demonstrable in vitro activity against HCV genotypes 1a, 1b, and 4 using different cell-based replicon assays.
Several amino acid substitutions at NS3 protease positions confer reducted activity, including F43, Q80, S122, R155, A156, and D168. The baseline prevalence of Q80K is 30% in genotype 1ainfected patients, but is less than 1% in genotype 1binfected patients. The Q80K polymorphism is associated with a lower sustained virologic response in clinical trials. Baseline determination of the Q80K polymorphism should be obtained before initiation of treatment with simeprevir.
Jean-Michel Pawlotsky, in, 2013
Retreatment Of Persons With Prior Peginterferon And Ribavirin Failure
The latest version of the AASLD-IDSA HCV Guidance no longer provides specific recommendations for retreatment of persons with a history of peginterferon plus ribavirin therapy, with or without an earlier generation direct-acting antiviral agent . The AASLD-IDSA HCV Guidance notes that these individuals respond to retreatment similar to treatment-naïve persons, thus implying the treatment approach should be the same as with treatment-naïve individuals. Although the pool of persons with a history of failure with a peginterferon-based regimen who need retreatment is small and diminishing, there are some individuals with this treatment history who need retreatment and may require special consideration that differs from that of treatment-naïve individuals. The following outlines a few of these key considerations based on available data and previous guidance that should be noted when retreating an individual with a history of prior treatment failure with peginterferon plus ribavirin, with or without an earlier generation DAA . Note that except for the 8-week option of glecaprevir-pibrentasvir , when retreating these individuals with first-line DAA combinations that have pangenotypic activity , the treatment will be the same as their treatment-naïve counterparts.
Learning Objective Performance Indicators
- List key factors that influence the choice and duration of therapy for treating HCV genotype 1 infection
- Describe the circumstances for NS5A genotypic drug resistance testing
- Discuss preferred therapies for initial treatment of treatment-naïve adults with HCV genotype 1 infection
- List the preferred therapies for retreatment of adults with chronic HCV genotype 1 infection who have previously failed therapy
You May Like: What Is Mild Hepatic Steatosis
Pegylated Interferon And Ribavirin
Regimens containing pegylated interferon and ribavirin were the backbone of HCV therapy from 2001 until 2014. Since 2014, interferon-free therapy has been the norm. However, interferon may still play a role in particular treatment situations . Ribavirin continues to play a key role in a wide variety of HCV treatment regimens.
Below, the term pegylated interferon refers to either peginterferon alfa-2a 180 µg subcutaneously once weekly or peginterferon alfa-2b 1.5 µg/kg SC once weekly. Note that peginterferon is contraindicated for use in patients with hepatic decompensation . Such patients may be at risk for worsening liver function or sepsis if treated with peginterferon.
Ribavirin is contraindicated in women who are or may become pregnant and in men whose female partner is pregnant this is due to the drugs potential teratogenicity.
Monotherapy with pegylated interferon, ribavirin, or any direct-acting antiviral agent is not recommended, due to their lack of efficacy.
Factors To Consider Prior To Choosing Retreatment Regimen
For persons with chronic HCV genotype 1 infection who have treatment experience, the key factors that influence the choice of the retreatment regimen are the prior regimen used when treatment failure occurred, the presence or absence of cirrhosis, and cost or insurance considerations. The retreatment of persons with HCV genotype 1 who have decompensated cirrhosis, severe renal impairment , or post-liver transplantation is not addressed here. For individuals with HCV-HIV coinfection, the approach to retreatment is the same as with HCV monoinfection, with the exception that additional drug interactions between DAAs and antiretroviral medications need to be taken into consideration and treatment duration differs in some circumstances.
Read Also: Is Hepatitis B Vaccine Live
Initial Treatment Of Hcv Infection
This section addresses treatment of patients with chronic hepatitis C who are naive to any type of therapy. Although regimens containing peginterferon and ribavirin plus direct-acting antiviral drugs are approved by the FDA for many HCV genotypes, the initial regimen for patients who are treatment-naive with HCV genotype 1 generally has been superseded by treatments incorporating regimens using only DAAs. Recommended treatments are viewed as equivalent, and the decision of which to use may involve consideration of drug interactions between the DAAs and concomitant medications . For example, the daily fixed-dose combination of ledipasvir and sofosbuvir has a potential interaction with proton pump inhibitors. Similarly, the daily fixed-dose combination of paritaprevir , ritonavir , and ombitasvir plus twice-daily dosed dasabuvir has a substantial interaction with the long-acting inhaled beta-adrenoceptor agonist salmeterol and other drugs that interface with the cytochrome P450 3A4 isoenzyme.
The New Generation Anti
Zepatier is FDA approved in May 2015 which is containing Elbasvir and Grazoprevir recommended for HCV genotypes including 1a, 1b as well as 4 without cirrhosis . According to the literatures, it has been revealed that initiation of treatment with this drug has SVR in 9497% of cases against genotype 1 and 97100% of cases against genotype 4. Moreover, the total of SVR for hepatitis without cirrhotic was 97% while it was 95.7% for cirrhotic . Mavyret is a FDA approved drug from 2017 which is recommended against wide range of HCV genotypes 26 without cirrhosis or with mild cirrhosis . In clinical trials, Mavyret was associated with SVR 92100% for HCV genotype 2 . Daclatasvir plus Sofosbuvir was appropriate for HCV genotypes 2 and 3 patients with SVR 94100% in different human trials . In a cohort study in Egypt, it has been shown that Daclatasvir plus Sofosbuvir dad achieved to SVR 96% for HCV genotype 4 infection after 12 months of treatment . Harvoni is as a RNA polymerase inhibitor which is the first FDA approved hepatitis therapeutic regimen free of interferon and Ribavirin . As noted it can improve the SVR rate from 94 to 99% against genotypes 1, 4, 5 and 6 . In the Fig. 1, some of effective anti HCV drugs and their effects on different compartments of HCV virus has been noted.
Also Check: How To Get Tested For Hepatitis C
Management Of Competing Interests
Members of the guideline panel have financial relationships with pharmaceutical companies related to HCV therapeutics. All members signed a commitment and competing interest statement at the outset of guideline development. Individuals with relevant disclosures were not excluded from voting on recommendations. However, in order to manage competing interests, the final guideline was vetted by the Canadian Association for the Study of the Liver membership, and specifically by the associations executive, to evaluate the presence of commercial bias. No funding, direct or in kind, was provided to the guideline panel for this work.
Also Check: Hepatitis A Vaccine San Diego Free
Management Of Hepatitis C Genotype 1 Monoinfection
Makia Dove, PharmD CandidateMonika N. Daftary, PharmD, BCPS, AAHIVPProfessorCollege of Pharmacy, Howard UniversityWashington, DC
US Pharm. 2014 39:75-79.
ABSTRACT: Hepatitis C is a common cause of end-stage liver disease in the United States. Historically, standard treatment for hepatitis C virus genotype 1 was peginterferon alfa plus ribavirin. Treatment remained unchanged until 2011, when the direct-acting antivirals boceprevir and telaprevir were approved by the FDA. Standard treatment of HCV genotype 1 now consists of peginterferon alfa, ribavirin, and a DAA. The addition of a DAA to standard treatment helped shorten the duration of therapy, but the initially approved agents were not without adverse effects . In late 2013, two additional DAAssimeprevir and sofosbuvirwere approved by the FDA. Both of these agents feature improved dosing-frequency and AE profiles.
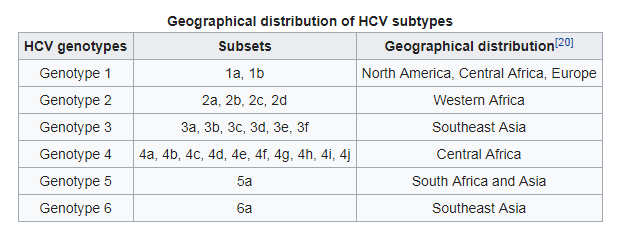
HCV is classified into six different genotypes , with each genotype divided into subtypes. HCV genotype 1, which is the most common cause of hepatitis C infection in the U.S., is the most difficult form to treat.4
You May Like: How Do You Get Hepatitis A
Pregnancy And Hepatitis C
Should pregnant women be tested for HCV antibodies?
Yes. All pregnant women should be screened for anti-HCV during each pregnancy, except in settings where the prevalence of HCV infection is < 0.1% . Pregnant women with known risk factors should be tested during each pregnancy, regardless of setting prevalence. Any pregnant women testing positive for anti-HCV should receive a PCR test for HCV RNA to determine current infection status.
Can a mother with hepatitis C infect her infant during birth?
The overall risk of an infected mother transmitting HCV to her infant is approximately 4%8% per pregnancy . Transmission occurs during pregnancy or childbirth, and no prophylaxis is available to protect the newborn from infection. The risk is significantly higher if the mother has a high HCV viral load, or is coinfected with HIV with which the rate of transmission ranges from 8%15% . Most infants infected with HCV at birth have no symptoms.
Should a woman with hepatitis C be advised against breastfeeding?
When should children born to HCV-infected mothers be tested to see if they were infected at birth?
How Genotypes Affect Treatment
Medications known as direct acting antivirals, or DAAs, stop the hep C virus from making copies of itself. Some DAAs appear to work well on all hepatitis C genotypes. Others work on only one or some.
Your doctor will probably prescribe some combination of these medications:
- Velpatasvir
Some pills combine two drugs into one pill.
You’ll probably take these meds for anywhere from 8 to 12 weeks. But they may not be right for everyone because of things like cost or other illnesses.
Your specific genotype can tell your doctor important things about how to use those medications, what to watch for, and other drugs you might need.
For example, you may have a higher chance for cirrhosis if you have genotype 1.
Genotype 3, the second most common subtype worldwide, may not respond as well to DAAs alone. In addition, this type might suggest that:
- Liver cancer is more likely.
- Insulin resistance might happen. When your body resists or doesn’t respond to insulin as well as normal, you have a higher chance of heart disease and diabetes.
- You might need longer, more challenging treatment
Your doctor might adjust or change your DAA treatment if you have:
Show Sources
American Association for the Study of Liver Diseases: âInitial Treatment of Adults with HCV Infection,â âHCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C.â
CDC: âHepatitis C Questions and Answers for the Public.â
Infohep.org: âHepatitis C treatment factsheet: Harvoni .â
Don’t Miss: Hepatitis B Titer Lab Test
Does Genotype Predict Response To Daa Therapy Like It Did For Interferon Therapy
Maybe maybe not.
Each of HCVs essential proteins work the same, regardless of genotype. These essential proteins may be structurally different due to small mutations.
Because theyre essential for the HCV life cycle, the structure of their active sites is least likely to change due to random mutation.
Because a proteins active site is relatively consistent between different genotypes, how well a particular DAA agent works is affected by where it binds on the target protein.
The effectiveness of those agents that bind most directly to the proteins active site is least likely to be affected by virus genotype.
All DAA drugs suppress ongoing HCV replication, but they dont eject the virus from its host cell. They also dont remove infected cells. This job is left to the persons immune system.
The variable effectiveness of interferon treatment indicates that the immune system is able to clear cells infected with some genotypes better than those infected by others.
Aside from genotype, there are many variables that can affect the likelihood of treatment success. Some of the more significant ones include:
- amount of HCV virus in your blood
- severity of liver damage before treatment
- the condition of your immune system
- age
- ongoing alcohol misuse
- response to prior therapies
Certain human genes can also predict how well treatment may work. The human gene known as IL28B is one of the strongest predictors of response to PEG/ribavirin treatment in people with HCV genotype 1.
- CC
- CT
- TT
Origin And Spread Of Hcv In The World

Epidemiological and phylogenetic studies have shown that the initial epidemic spread of HCV in Japan occurred in the 1920s1930s through mass campaigns of parenteral antischistosomal therapy , followed during World War II by intravenous drug use , transfusion, and unsafe invasive medical and surgical procedures. Similarly, in Europe, the initial spread of the virus started during the last century through the use of unsafe parenteral injections, invasive medical and surgical procedures and transfusion of blood products. An epidemic explosion of IDU shortly followed the iatrogenic spread in the early 1960s both in the United States and Europe .
The divergence of subtype variants is estimated to have occurred less than 100 years ago, whereas the numerous subtypes of HCV are proposed to have diverged some 300 years ago while the major HCV genotypes would have originated at least 5002000 years ago.
Recommended Reading: Why Are Baby Boomers Being Tested For Hepatitis C
Don’t Miss: What Is A Hepatitis Panel
Study Design And Population
This nationwide cohort study included patients infected with a nonepidemic HCV genotype treated with an interferon-free DAA regimen. Nonepidemic HCV genotypes were defined as genotypes and subtypes other than 1a/1b/2a/2b/3a/4a/4d. All laboratories performing HCV genotyping in the Netherlands were approached. All but 1 participated in the study: the Amsterdam University Medical Centers Sanquin Diagnostics, Amsterdam UMC Groningen, Groningen LUMC, Leiden Erasmus Medical Center, Rotterdam and Maastricht UMC, Maastricht.
What Is The Current Research Into Genotypes And Treatments For Each Type
The most widely used anti-HCV therapy, PEG/ribavirin, doesnt target the virus itself. This treatment regimen primarily affects the persons immune system. Its goal is to rally the immune system to recognize and eliminate cells infected with HCV.
However, variations of HCV in a single person wont necessarily look the same to the immune system. This is one of the reasons that HCV infections persist and become chronic infections.
Even with this genetic diversity, researchers have identified proteins that are required for the reproduction of HCV in the body. These proteins are present in essentially all of the many HCV variants.
The new treatments for HCV target these proteins. That means they target the virus. Direct-acting antiviral therapy uses small molecules designed to specifically inhibit these viral proteins.
Many DAA drugs have been under development during the past decade. Each drug targets one of the handful of essential HCV proteins.
The first two DAA drugs, boceprevir and telaprevir, got approval for use in the United States in 2011. Both target a particular type of HCV enzyme known as protease. These drugs are used in combination with PEG/ribavirin.
Both of these new medications are most effective for HCV genotype 1. Theyre moderately effective for genotype 2, and not effective for genotype 3.
Initially, they were only approved for use in people with genotype 1 HCV in combination with PEG/ribavirin.
Read Also: Hepatitis C Is It Curable
Ns3 Amplification Library Preparation And Ngs
Table 2.
HCV-specific oligonucleotide sequences used for amplification and sequencing of NS3 protease
A threshold value 70% was used to delineate a distinct consensus sequence belonging exclusively to the clade I or clade II genotype . Samples with mapping values below this threshold were considered as mixed samples and 2 different consensus sequences were retrieved . Consensus sequences were called by combining SUPER-CAP and SAMtools programs , and used in MEGA 7 software to create a maximum likelihood phylogenetic tree based on the general time reversible substitution model which was selected by best model search using the Find best DNA Model function implemented in MEGA7 . RASs were analyzed using 1, 5, 10, 15, and 20% cutoffs.
Fig. 1.
Phylogenetic tree showing evolutionary relationships between our HCV subtype 1a sequences and reference isolates included in this study. The evolutionary history was inferred using the maximum likelihood method based on the GTR+G+I as the best-fit model of evolution. Our viral isolates are highlighted with colored circles: red yellow pink blue light blue green violet teal orange . GenBank accession numbers for reference clade I and clade II isolates are also shown.
Clinical Significance Of Hepatitis C Genotypes
Genotype generally has not been found in epidemiological studies to play a large rolein liver disease progression due to HCV. Rather, genotype is of clinical importanceprincipally as a factor in selecting the appropriate HCV medications for treatment. Please see the HCV Treatment Considerations for more information.
Recommended Reading: Echogenic Liver Consistent With Hepatic Steatosis
Ns3 Sequencing Resistance Mutations And Genetic Barrier
Sanger NS3 sequencing for the plasma samples from the Italian sites was performed as previously described . Samples from the German sites were processed, amplified by reverse transcription polymerase chain reaction, and sequenced as previously described . The Italian and German sequences have been submitted to GenBank Genotyping and subtyping was based on phylogenetic analysis of the same NS3 sequence .
NS3i resistance-associated mutations were analyzed based on the latest IAS-USA reference list . The genetic barrier was analyzed for the codons associated with resistance mutations. For each codon, we calculated an arithmetic sum score for the smallest number of transitions and/or transversions required for evolution to the target resistance mutation, counting 1 for transitions and 2.5 for transversions .
The clade-specific genetic barrier was computed as the weighted average score for all the codon variants found in the sequences of that clade and different from that coding for the target resistance mutation.
The First Hcv Protease Inhibitors
In May 2011, the HCV NS3/4A protease inhibitors boceprevir and telaprevir received FDA approval for patients infected with HCV genotype 1. However, treatment with either of these agents is no longer recommended because of the higher efficacy and improved safety profile of other regimens. The sale and distribution of telaprevir was discontinued in the United States in 2014. The sale and distribution of boceprevir was discontinued in the United States in 2015.
A third protease inhibitor, simeprevir, received FDA approval in November 2013 for use in patients infected with HCV genotype 1. Initial approval specified that simeprevir be used in combination with peginterferon and ribavirin in patients with compensated liver disease. However, the naturally-occurring NS3/4A Q80K amino acid substitution was problematic for patients with genotype 1a. This resistance-associated variant was seen in about 30% of patients with genotype 1a infection. If present, the polymorphism was associated with a lower SVR in simeprevir-treated patients. SVR rates in previously untreated patients were reported as 84% in genotype 1a patients without the Q80K polymorphism, 58% in genotype 1a patients with the Q80K polymorphism, and 85% in genotype 1b patients. Accordingly, it was recommended that patients with genotype 1a and the Q80K polymorphism not receive combination therapy with peginterferon, ribavirin, and simeprevir.
Don’t Miss: Hepatitis B Test Kit Walgreens