Other Things To Know:
- The viral load measurement does not tell us anything about the severity of a patients liver disease or the degree of fibrosis . For that information, the patient would need additional testing.
- It is not necessary to check the viral load repeatedly during treatment.
- If a quantitative HCV RNA result is reported as < 15 IU/L, this means that the quantitative test cannot measure the hepatitis C virus. It may mean that there is no detectable HCV RNA at all, but it may mean that the level of virus is just too low for the test to pick it up.
You May Like: Does Hepatitis Affect The Liver
Baseline Characteristics And Hcc Risk
In casecohort analysis, the risk of HCC was increased starting at the fourth quintile of HBV viral load , as compared with the lowest quintile of viral load. HBeAg positivity, genotype C HBV infection and elevated ALT activity were also associated with increased risk for HCC .
In subcohort analysis, men infected with HBV genotype C had a higher cumulative incidence of HCC than men with B or B plus C mixed genotype infection . The cumulative incidence of HCC within 10 years was 14.49% for genotype C subjects and 2.14% for other genotype subjects. The difference in the cumulative HCC incidences between the two groups was greater if baseline viral load was 4.39 log copies/ml and lesser if baseline viral load was < 4.39 log copies/ml .
KaplanMeier estimates of the cumulative incidences of HCC by HBV genotype. The analysis was based on the subcohort randomly selected from the entire cohort.
What Do Viral Load Test Results Mean
Viral load testing can be confusing. The results do not always predict symptoms or the amount of damage HCV may be doing to your liver. A high viral load doesnt mean that you are getting worse. If your viral load is lower than a previous test, and you are not on HCV treatment, it does not mean that you are getting better.
When you have a viral load test, the results are typically reported in international units per milliliter and copies per milliliter. A viral load is considered:
- High if greater than 800,000 IU/mL
- Low if less than 800,000 IU/mL
Read Also: Is Hepatitis C Contagious Airborne
Recommended Reading: Hepatitis C Treatment Side Effects
General Characteristics Of The Study Population
A total of 228 patients with HIV were analyzed most of them were men between 32 and 46 years of age . At the time of this study, 82.0% of the participants were on antiretroviral therapy, with a median HIV viral load of 40.0 IU/mL . Among the 187 treated patients, the medical records revealed that cART was tailored individually in which none were monotherapy. All received at least one drug that is known to affect the HBVs life cycle, such as tenofovir, lamivudine, or emtricitabine. However, the most frequent combinations were tenofovir + emtricitabine + efavirenz in 39.6% , followed by tenofovir + emtricitabine + lopinavir + ritonavir in 11.2% and 49.2% received diverse unique combinations with these and other antiviral drugs. Also, 62 individuals out of 132 had evidence of hepatitis C virus infection . Overall, liver injury was detected in 24.6% , of which 18.3% had significant liver fibrosis, 6.9% had advanced fibrosis, and with liver stiffness, 22.5% had advanced liver fibrosis .
Table 1. Demographic data of patients with HIV and HIV/HBV.
Table 2. Baseline clinical characteristics of patients with HIV and HIV/HBV.
Table 3. Baseline non-invasive markers of liver damage in HIV and HBV/HIV patients.
How Can We Help
Hepatitis C test only require basic blood sample, which can also be collected from help to get the test done and to help you do so is our highly reliable and well established at home healthcare services, wherein you can not only get Hepatitis C home test but, can also get doctors consultation at home. So, you neednt have to worry of getting tested, collecting the hepatitis test reports and then running to a doctors. As we have designed our at home healthcare services keeping the comfort of patients at mind. So, if you need Hepatitis C home test done or hepatitis C treatment at home or any other healthcare services at home just give us a call and we will be there at your service.
You May Like: Can You Get Hepatitis From Saliva
Distribution Of Hbv Genotypes And Mixtures
Among the 48 patients with detectable HBV viral load, 25 cases were positive to the nested PCR assay in which 44 HBV genotypes were detected by multiplex PCR . According to our methodology, the minimum viral load for HBV genotyping was 82.0 IU/mL . The predominant HBV genotype was H , followed by G , D , A , and F . Figure 2C illustrates the phylogenetic tree built for each genotype of the 38 strains confirmed by direct DNA sequencing. All genotypes A were sub-genotyped as A2, one genotype F was classified as F1b, and one D was D4. Among typeable samples, 44.0% had a single genotype , whereas 36.0% had dual-mixtures , two were D/H, two were A2/H, and one was A2/G) Moreover, 20.0% were triple-mixtures . G/H and H/G/D were the most frequent genotype mixtures. Nineteen out of 25 genotyped cases were under cART in which five mono-infected, three dual-mixed, and one triple-mixed were treated with tenofovir + emtricitabine + efavirenz. Two triple-mixed cases were treated with tenofovir + emtricitabine + lopinavir + ritonavir whereas three mono-infected, four dually infected and one triple-mixed case received diverse combinations. Interestingly, ten patients had high HBV viral loads despite receiving a tenofovir-based regimen five were mono-infected, , one dual-mixed , and four were triple-mixed .
Assessment Of Liver Damage
Liver fibrosis was assessed through three non-invasive methods: transitional elastography , Aspartate aminotransferase to Platelets Ratio Index score , and Fibrosis-4 score . TE was carried out using a FibroScan® instrument . Liver stiffness measurement expressed in kiloPascals was performed by the same qualified physician. LSM was considered reliable if ten successful measurements were obtained, and the success rate was > 80%. APRI was calculated considering the upper limit of normal serum AST for men equal to 40 IU/in the equation AST IU/L/ ×100 . FIB-4 was calculated with the equation / 1/2IU/L) . APRI cut-off value 0.7 was considered significant hepatic fibrosis, while FIB-4 cut-off value 3.25 was interpreted as advanced fibrosis . Also, LSM was classified as F1 , F2 , F3 , and F4 or cirrhosis . F1-F2 was taken as mild liver fibrosis and F3-F4 as advanced liver fibrosis . Levels of liver enzymes, platelets, HIV viral load, CD4, and CD8 lymphocytes were attained by routine analysis from the hospitals Central Laboratory.
Don’t Miss: Hepatitis C And Liver Damage
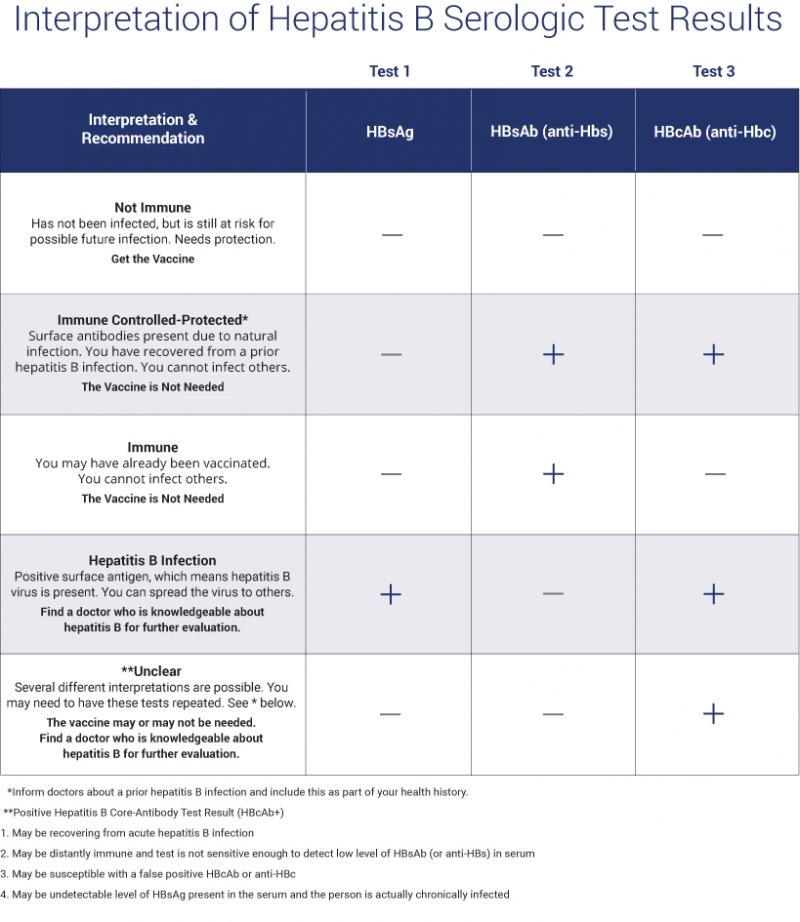
Diagnosed With Chronic Hepatitis B What Does Your Hbv Dna Test Tell You
If you have been diagnosed with chronic hepatitis B, your doctor has probably run several blood tests that show if the infection is harming your liver and identify what stage of infection you are in. Doctors consider all of these results when deciding if you need treatment and how often you should be monitored.
In this blog, well examine how one of the tests the HBV DNA or viral load test can give you a snapshot into your hepatitis B infection and your health. The HBV DNA test is performed on a blood sample using a Polymerase Chain Reaction technique that rapidly generates HBV DNA fragments so they can be measured. Today, viral load is usually measured using international units per milliliter . However, in the past it was measured in copies per milliliter , and in some regions and labs, it is still used.
If you ever need to convert copies into international units, there are about 5.6 copies in one international unit, so 5,000 copies/mL equals about 893 IU/mL. Remember to keep copies of your lab information on file so you can track your status. An Excel spreadsheet works great.
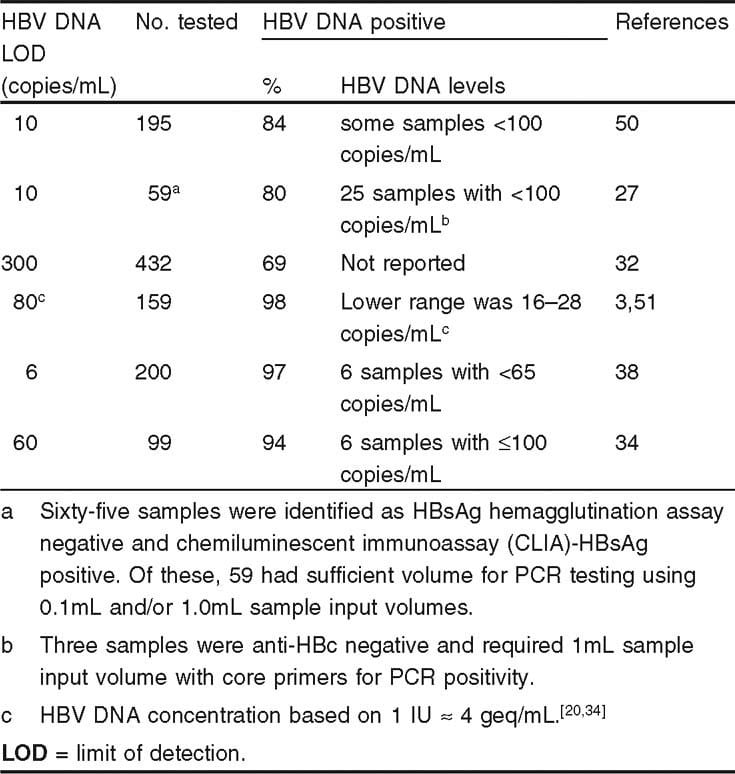
The sensitivity of HBV DNA tests may vary with each lab so its a good idea to use the same lab for your test. Labs usually measure down to less than 200 IU/mL. Below the threshold, the viral load is considered undetectable something everyone with chronic hepatitis B wants to hear.
Hepatitis Serologic Analysis And Hbv Dna Assay
HBV serologic markers, including HBsAg, HBsAb, HBeAg, HBeAb, anti-HCV Ab , antiâhepatitis-D antibody, and anti-HIV antibody, were tested by using commercially available enzyme immunoassays . HBcAb was tested by radioimmunoassay . Serial serum samples from the same patient were tested in a single run to minimize interassay variation. A nested PCR assay for detection of serum HBV DNA was done by using primer sets from the HBV surface antigen and the core antigen coding region. For the surface region, the primers were CCTGCTGGTGGCTCCAGTTC and CAAACGGGCAACATACCTTG for the first round of PCR testing and ACATCAGGATTCCTAGGACC and CGCAGACACATCCAGCGATA for the second round. The corresponding sets of primers for the core region were GGAGTGTGGATTCGCATCCTCC , ATACTAACATTGAGATTCCC , AGACCACCAAATGCCCCTAT , and GATCTTCTGCGACGCGGCGA . Using serial dilution of EuroHep-2 HBV DNA plasma standards, we estimated the detection limit of the PCR assay to be 1âÃâ102 genomes/mL. Results of the Digene Hybrid Capture II assay were expressed in copies per milliliter, and the manufacturer’s stated cut-off limit for detecting HBV viremia in clinical specimens was 0.142âÃâ106 copies/mL. Reverse transcriptionânested PCR for HCV was done in a single tube assay as described previously. The outer primers 57 and 321 and the inner primers 126 and 299 were designed from the highly conserved 5â² noncoding region.
Also Check: Hepatitis B Liver Cancer Treatment
Pretransplantation Characteristics Associated With Hbv
Pretransplantation characteristics associated with hepatitis due to HBV reactivation after HCT were an elevated serum ALT level, HBsAg positivity, detectable serum HBV DNA , and BCP . Three patients with elevated serum ALT before HCT and 10 patients with normal serum ALT before HCT had hepatitis due to HBV reactivation . Eleven patients who were positive for HBsAg before HCT had hepatitis due to HBV reactivation, where only 2 HBsAg-negative patients had hepatitis due to HBV reactivation . Nine patients with detectable serum HBV DNA and only 4 without detectable serum HBV DNA had hepatitis due to HBV reactivation . Pretransplantation serum HBV DNA levels were also significantly higher in patients with hepatitis due to HBV reactivation than in those without hepatitis due to HBV reactivation . In addition, 7 patients with BCP and 5 without BCP had hepatitis due to HBV reactivation .
Breaking Down The Numbers
- Low viral load. A viral load of less than 800,000 IU/mL is considered low. Successful treatment is more likely with a low viral load.
- High viral load. A viral load of more than 800,000 IU/mL is considered high. This can make successful treatment more challenging.
- Undetectable viral load. A viral load of less than 615 IU/mL means theres no detectable HCV, or its too low to detect.
During treatment, a falling viral load is an indication that treatment is succeeding.
At the end of the planned course of treatment, which is generally 8 to 12 weeks , an undetectable viral load means that treatment can be stopped.
A sustained virologic response is when the most sensitive tests find no trace of HCV 12 weeks after stopping treatment. After that, viral load testing can alert you to a relapse.
Read Also: How Do You Get Infected With Hepatitis B
Sana Irfan1* Mahua Das Gupta2 Np Sahu3 Zulfiquar Ali Bhuttoo4 And Poonam Kumari4
*Corresponding author:Received:Accepted:Keywords
Cite this as
Abstract
Background: Quantification of the viral burden is an important laboratory tool in the management of hepatitis B virus -infected patients. However, widespread use of assays is still hampered due to the high cost. Treatment reduces viral load to undetectable levels. HBV infected patients tend to have high HBV DNA levels, and severe liver disease.
Objectives: This study was carried out to determine the pattern of HBV viral load levels of patients in Jharkhand.
Method: Variables included socio demographics like age, sex, occupation, history of vaccination, surgery, IVDU.etc. The COBAS Amplicor automated Analyzer was used to assay the virus quantitatively.
Results: 366 number of patients were tested from 2013 to 2015. HBV viral titre ranged between. < 6 IU/ml to> 1.1 × 108.
There was a high occurrence of viral titre as well as suspected core mutants in the population studied. Viral load is a risk factor for cirrhosis and hepatocellular carcinoma. A policy earmarked to combat this virus in Jharkhand is hereby solicited.
Main article text
How Often Do I Need A Viral Load Test
Understanding the specifics of your viral load is important at the time of diagnosis. Once you begin treatment, follow-up testing will let your doctor know if the current treatment is effective.
Other than that, theres no need for repeat testing. This is because the viral load doesnt provide information about your symptoms or whether your liver is functioning properly. Other liver tests, such as a biopsy, can provide that information.
Certain groups are more vulnerable to contracting HCV. Among them are:
- people on dialysis
- children born to HCV-positive mothers
- anyone who may have had contact with the blood of someone with hepatitis C
The most common methods of HCV transmission are:
- sharing needles and syringes used for injecting drugs
- a mother with hepatitis C transferring HCV to her child during childbirth
Occasionally HCV is transmitted through:
- having sex with someone who has hepatitis C
- getting a tattoo in a place that doesnt have good infection control
- sharing personal care items, such as a razor or toothbrush, with someone who has hepatitis C
Hepatitis C cant be transmitted through:
- coughing or sneezing
You May Like: Can You Spread Hepatitis C
Who Is Most At Risk Of Contracting Hepatitis C
You have a high risk of contracting hepatitis C if you:
- use or have used injection drugs even if it was just once or many years ago
- have received blood or blood products or an organ transplant before July 1990 in Canada
- have been in jail or
- have been injected or scratched during vaccination, surgery, blood transfusion or a religious/ceremonial ritual in regions where hepatitis C is common.
You have a high moderate risk of contracting hepatitis C if you:
- have tattoos or body piercing
- have multiple sexual partners
- have a sexually transmitted infection , including HIV or lymphogranuloma venereum
- have experienced traumatic sex or rough sex or have used sex toys or fisting that can tear body tissue
- have vaginal sex during menstruation
- have received a kidney treatment
- have received an accidental injury from a needle or syringe
- have another infectious disease
- were born to a hepatitis C infected mother or
- have a sexual partner infected with hepatitis C.
Hepatitis C is NOT passed from person to person by:
- coughing, sneezing
- breastfeeding unless your nipples are cracked and bleeding or
- oral sex, unless blood is present.
Recommended Reading: How Long Is A Hepatitis C Shot Good For
Measuring Viral Activity Tells Us How Effectively A Treatment Is Working
A viral load is simply the measurement of the amount of virus in your blood. Viral load measurements are commonly used to monitor chronic viral diseases such as HIV, hepatitis B , and hepatitis C .
In the case of HCV, a test called a quantitative HCV RNA assay is used to measure the viruss genetic material detected in a milliliter of blood. Other technologies can be also used to monitor viral activity, most of which do so by detecting either viral DNA or RNA.
Also Check: The Difference Between Hepatitis B And C
Predictive Value Of The Hbv Rna Level For The Virologic Response In Chb Patients Treated With Nas
The serum HBV RNA level was significantly lower at week 12 , week 24 , and week 48 in patients with a VR than in those without a VR . However, the serum RNA level was similar and non-significant between the two groups at baseline .
The HBV RNA level 12 weeks after treatment had a certain predictive value for a VR at 96 weeks after treatment . The HBV RNA level 12 weeks after treatment was an independent predictor for a VR , with a cutoff value of 9.05 log10 copies/mL .
|
Table 3 Univariate and Multivariate Analyses of Associations Between the HBV RNA Level and Virologic Response at Week 96 |
Qualitative Detection Of Hbv Dna
The yield of DNA extracts was tested by nested PCR. The amplification reaction was prepared with 5 L of DNA, 1 L of each primer , 0.5 L of dNTPs mix , 1 L of MgCl2 , 2.5 L of PCR buffer , 13.8 L of water and 0.2 L of Taq DNA polymerase in a final volume of 25 L. The first round amplified a 418-bp fragment of the surface gene under the following conditions: 94°C for 3 min followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, with a final extension step of 72°C for 5 min. The second round amplified a 232-bp product under the same conditions.
Also Check: How Is Hepatitis B And C Transmitted
How Is A Person Tested For Hepatitis C
A viral-load test is used to check for hepatitis C in the bloodstream. Usually, hepatitis C virus can be found in a persons bloodstream two weeks after he or she becomes infected.
*Except in case of recent risk or in people with a weakened immune system**During the first six months after HCV infection, a person may spontaneously clear the virus if there was a recent risk, repeat viral-load testing to confirm chronic hepatitis C infection