Interferon Signaling And Treatment Outcome In Chronic Hepatitis C
See allHide authors and affiliations
- For correspondence:
Edited by Charles M. Rice III, The Rockefeller University, New York, NY, and approved March 6, 2008
Case 1 Induction Of Autoimmune Hepatitis By A Course Of Peginterferon And Ribavirin For Chronic Hepatitis C
Key Points
| < 1.2 |
A patient with HBeAg positive chronic hepatitis B developed an acute flare of disease and jaundice within 4 weeks of starting interferon alfa therapy. The flare was accompanied by a dramatic decrease in HBV DNA levels, followed by clearance of both HBeAg and HBsAg and resolution of the chronic hepatitis. Thus, the liver injury reflected clearance of hepatitis B virus rather than direct hepatotoxicity of interferon. An acute flare of hepatitis is often considered a favorable prognostic sign during the therapy of chronic hepatitis B presaging loss of HBeAg and, sometimes, loss of HBsAg as well. The initial HBV DNA testing was based upon a relatively insensitive method which had a lower limit of detection of 160,000 copies/mL. In follow up, polymerase chain reaction based assays for HBV DNA were used that had a lower limit of detection of 500 copies/mL.
Using Pegylated Interferon And Ribavirin To Treat Patients With Chronic Hepatitis C
RAYMOND P. WARD, M.D., PH.D., St. Marys Family Practice Residency, Grand Junction, Colorado
Am Fam Physician. 2005 Aug 15 72:655-662.
Hepatitis C virus infection is the most common chronic blood-borne infection in the United States, with en estimated 3.8 million persons exposed and 2.7 million persons chronically infected.1 The incidence of new infections was greatest from the 1960s through the 1980s, peaking at around 250,000 new cases annually, and has now dropped to 30,000 to 40,000 new cases annually.2 The most common routes of infection are injection drug use and previous contact with tainted blood products. This disease usually runs an asymptomatic course for many years, and some patients never develop any symptoms. If symptoms do develop, they often are nonspecific . About 10 to 20 percent of chronically infected persons progress to cirrhosis over an average of 20 years.24 Faster rates of progression are seen in persons who are infected at an older age, or who have modifying risk factors such as moderate or heavy alcohol consumption, human immunodeficiency virus , or other coexistent liver diseases.3,4
A = consistent, good-quality patient-oriented evidence B = inconsistent or limited-quality patient-oriented evidence C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, see page 555 orwww.aafp.org/afpsort.xml.
You May Like: Hepatitis C Drugs In India
Patient Evaluation And Treatment
Recommendations
Treatment should be considered for all patients with detectable HCV RNA and an abnormal liver biopsy, regardless of the presence or absence of liver enzyme elevation.
Prior to making a decision regarding treatment, patients should be evaluated with HCV RNA, HCV genotype, liver enzymes , and liver biopsy, unless contraindicated. The decision to initiate antiviral therapy should be made based upon the willingness of the patient to undergo therapy, ability to regularly attend appointments, and agreement to use contraception to prevent pregnancy. The decision to initiate antiviral therapy should be made on an individualized basis that considers severity of liver disease, co-morbid conditions, the potential for serious side effects and the likelihood of response.
Patients with HCV infection on methadone maintenance therapy should not be considered ineligible for treatment.
The treatment of the actively using injection drug user is not contraindicated and may be appropriate under some circumstances. Patients with a history of well-controlled psychiatric disorders may be excellent candidates for antiviral therapy and should be under the care of a qualified mental health professional.
All patients with CHC infection are candidates for antiviral therapy. These patients are defined by detectable serum HCV RNA and an abnormal liver biopsy consistent with chronic liver disease. Treatment is recommended for patients with significant inflammation or fibrosis.23
Combination And Sequential Treatment With Nas
Many early studies combining lamivudine and PEG-IFN failed to show any convincing evidence of benefit. Several recent studies have explored the use of combination and in sequential therapy with the latest generation NAs therapy, ETV and TDF.
A reason for the lack of a superior response from combination therapy may be that a longer treatment duration is required. The effectiveness of extended treatment was investigated in a study conducted in China, where 47 patients were treated with 96 weeks of PEG-IFN-2a in combination with LAM or adefovir disoproxil . At 6 months post treatment, this strategy achieved high rates of HBeAg and HBsAg seroconversion of 72.3% and 27.7%, respectively. The results should be interpreted with caution as this was a small study with no PEG-IFN monotherapy arm for comparison .
In the study of telbivudine plus PEG-IFN-2a, a rapid and profound reduction in HBV DNA levels was reported . However, the combination of PEG-IFN and telbivudine was found to be associated with an increased risk of peripheral neuropathy, which resulted in early trial termination. Thus this combination is not recommended.
Read Also: Best Food For Hepatitis C
Antiviral Medication For Hepatitis B
Doctors may recommend antiviral medication for people with chronic hepatitis B, which occurs when the virus stays in your body for more than six months.
Antiviral medication prevents the virus from replicating, or creating copies of itself, and may prevent progressive liver damage. Currently available medications can treat hepatitis B with a low risk of serious side effects.
NYU Langone hepatologists and infectious disease specialists prescribe medication when they have determined that without treatment, the hepatitis B virus is very likely to damage the liver over time. People with chronic hepatitis B may need to take antiviral medication for the rest of their lives to prevent liver damage.
There are many different types of antiviral medications available, and your doctor recommends the right type for you based on your symptoms, your overall health, and the results of diagnostic tests. A doctor may take a wait-and-see approach with a person who has a healthy liver and whose blood tests indicate a low viral load, the number of copies of the hepatitis B virus in your bloodstream.
Someone with HIV infection or AIDS may have a weakened immune system and is therefore more likely to develop liver damage. The U.S. Centers for Disease Control and Prevention strongly recommends that people with HIV infection who are diagnosed with hepatitis B immediately begin treatment with antiviral medication.
Interferon For Interferon Nonresponding And Relapsing Patients With Chronic Hepatitis C
The clinical data were limited to patients with histologic evidence of severe fibrosis who were retreated with pegylated interferon. In this scenario, retreatment with interferon did not appear to provide significant clinical benefit and, when only the trials at low risk of bias were considered, retreatment for several years may even have increased all-cause mortality. Such treatment also produced adverse events. On the other hand, the treatment did result in improvement in some surrogate outcomes, namely sustained viral responses and histologic evidence of inflammation. Interferon monotherapy retreatment cannot be recommended for these patients. No clinical data are available for patients with less severe fibrosis. The sustained viral response cannot be used as a surrogate marker for hepatitis C treatment in this clinical setting with low sustained viral response rates and needs to be validated in others in which higher sustained viral response rates are reported.
To assess the benefits and harms of interferon monotherapy retreatment in chronic hepatitis C patients and to validate the currently employed surrogate outcomes in this group of patients.
We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expandeduntil 16 August 2012.
Recommended Reading: How To Manage Hepatitis C
Correlation Between Responsiveness To Pr And Daas
To establish the correlation between the responsiveness of chronically HCV infected individuals to PR and DAAs, we collated data from all clinical trials that reported SVR rates achieved with DAA-based treatments in treatment-naïve individuals, SVRnaive, and in previous null responders to PR, SVRnull . The data are grouped according to treatment regimen and summarized in Table 1. Individual datasets are listed in S1 Table. We found that SVRnaive> SVRnull with P1059 overall . The difference was starker when the analysis was restricted to treatments that included PR , but, importantly, was highly significant when interferon-free regimens alone were considered . The difference remained when only individuals with or without liver cirrhosis were considered or when the analysis was restricted to studies that did not factor liver cirrhosis . The difference was clearer for treatments that elicited < 100% SVR than for more recent, stronger treatments that elicited ~100% SVR regardless of treatment experience. Nonetheless, the clinical evidence of a positive correlation between responsiveness to PR and DAAs was overwhelming and suggested a causal relationship between the two. We proposed a mechanistic hypothesis underlying this relationship, where greater IFN-responsiveness exerted better control on RAVs and improved DAA treatment outcomes , and constructed a mathematical model to test it.
Nucleoside And Nucleotide Hcv Polymerase Inhibitors
SOF is currently the only approved nucleotide inhibitor and has been approved in January 2014. SOF can be used in combination with peg-IFN and RBV for 12 weeks, leading to SVR rates of 90% in patients with HCV GT1 infection, and with RBV alone for 12 weeks for GT2 as well as 24 weeks for GT3 . IFN-free SOF-based therapies of GT1 should always include a combination with other DAA. Further substances with this pan-GT DAA activity are currently under development, e.g. MK-3682 , in a phase I/IIa study.
Don’t Miss: Does Hepatitis C Go Away
E Treatment Of Hepatitis C
The primary goal of HCV therapy is to achieve a SVR, defined as an undetectable HCV RNA 6 months after stopping antiviral therapy. Secondary goals of antiviral therapy include improvements in histology, quality of life and prevention of hepatocellular carcinoma. Antiviral therapy is approved by the Food and Drug Administration for patients with persistently abnormal liver enzymes, detectable HCV RNA and an abnormal liver biopsy. Recent data have shown that patients with normal liver enzymes, detectable HCV RNA and an abnormal liver biopsy respond to therapy at similar rates as those with abnormal liver enzymes.55
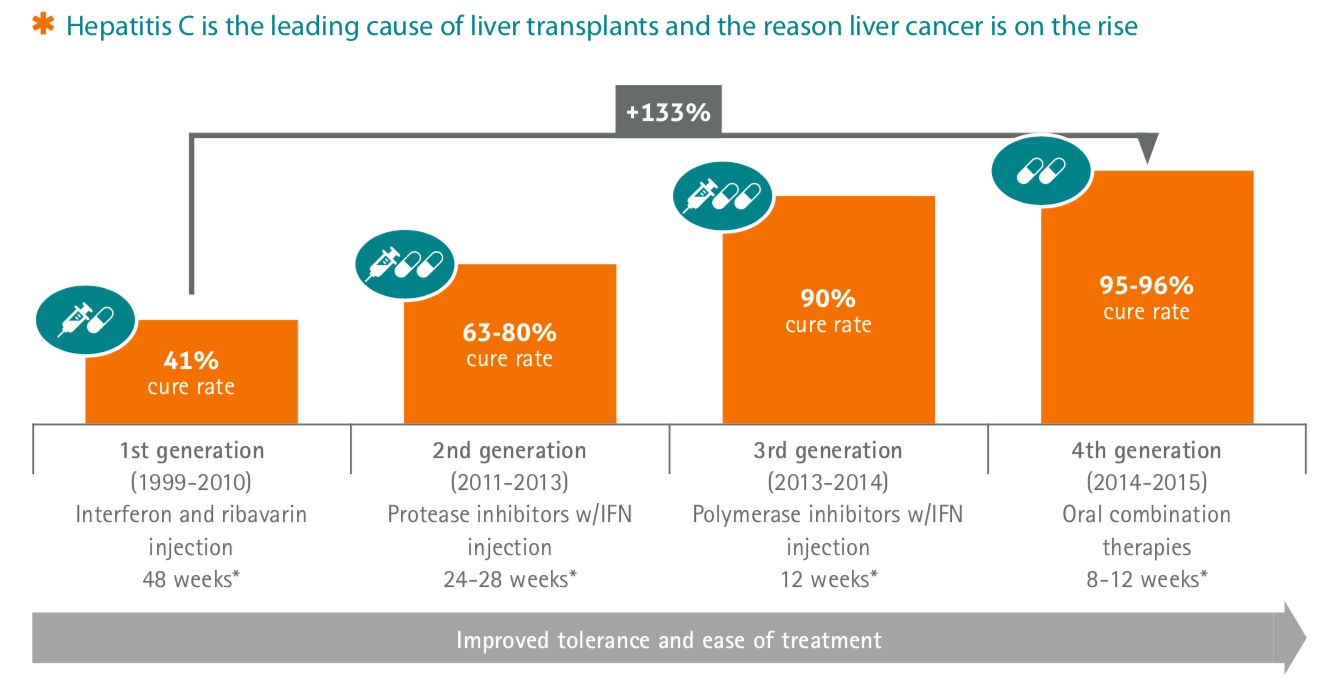
The efficacy of HCV treatment has improved over the past decade. Initial treatment consisting of interferon alpha has been replaced by pegylated interferon and now by combination therapy using pegylated interferon and ribavirin. Efficacy varies depending on multiple factors especially viral genotype, but achieving sustained viral suppression in 50% of patients can be expected .
A Subset Of Genes That Predicts Response To Treatment
Supervised classifier analysis of array data allows the identification of a subset of genes that best predicts the outcome, in our case rapid response vs. nonresponse at week 4. All liver biopsy and PBMC datasets were subjected to supervised classifier prediction using the response at 4 weeks of treatment as grouping criteria. For PBMC samples, the analysis did not identify a subset of genes that could predict the treatment outcome . In contrast, a subset of 16 genes was identified in the liver B-2 samples that predicted response to treatment with an error rate of 16.1% using the K Nearest Neighbors test . An even better prediction was possible with a subset of 29 genes in the pretreatment biopsies B-1, where the error rate was 4.3% . In this set, there were 22 genes up-regulated by pegIFN2b . Therefore, 76% best predictor genes represent ISGs.
Contrary to the predominance of ISGs in the best predictor set from pretreatment biopsies, only 3 of the 16 best predictor genes derived from an analysis of the B-2 biopsies were ISGs . These results support the findings shown in that expression levels of most ISGs in B-2 do not differ between RR and non-RR samples and therefore are not suited for the discrimination of responders from nonresponders. Among the non-ISGs present in the B-1 and B-2 liver biopsy lists discussed above are genes having functions in signal transduction, cell cycle regulation, apoptosis, and amino acid metabolism.
You May Like: Can Hepatitis C Go Away On Its Own
Are There Any Differences Among The Different Types Of Interferons
Although interferons are very similar they affect the body differently. Therefore, different interferons are used for different conditions.
- Interferon alphas are used for treating cancers and viral infections
- interferon betas are used for treatingmultiple sclerosis and
- interferon gamma is used for treating chronic granulomatous disease.
Interferon And Ribavirin For Chronic Hepatitis C: Should It Be Administered In The New Treatment Era
1Department of Pharmacy, Laboratory for Teaching and Research in Social Pharmacy.2Department of Medicine.3Department of Physiology, Federal University of Sergipe UFS, São Cristóvão, SE, Brazil.
Acta Gastroenterol Latinoam 2017 47:14-22Recibido: 28/09/2015 / 29/09/2016 / Publicado en www.actagastro.org el 05/04/2017
Summary
Key words. Hepatitis C, pharmacotherapy, interferon, ribavirin, treatment.
Don’t Miss: How Does Hepatitis C Affect The Liver
Treatment Of Hepatitis C Infections With Interferon: A Historical Perspective
Sara Contente
1Department of Pathology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA
Abstract
Interferons were first described in 1957, but it was not until 34years after their discovery that sufficient quantities of it wereavailable for treatment of hepatitis C virus infections,Clinicians now have an excellent understanding of the basis forthe effectiveness of interferon alpha in the therapy ofthis disease. Treatment with IFN- is more efficient when itcomplemented by the antiviral ribavirin and the IFN- is conjugatedwith polyethylene glycol to form peginterferon. In the near futuretreatment of HCV with IFN- may involve new anti-HCV agents thatare currently under development.
Interest in IFNs was reignited in the mid-1970s when sufficient quantities of fairly clean human IFN-, obtained by Cantellâs group in Finland from the white blood cell buffy coats of donated blood, became available for clinical experiments. Many of these had promising, if not highly significant, results in studies on the prevention of common colds and the treatment of several herpes virus infections, such as herpes keratoconjunctivitis and the varicella-zoster infections, shingles and chickenpox . The discovery that in tissue culture experiments mouse IFN- inhibited chronic infections with mouse leukemia viruses prompted additional studies employing interferon as therapy for human chronic hepatitis B virus infections. These had very promising results .
References
Measurement Of Ifn In Serum
Pretreatment IFN levels and the concentration of pegIFN2b 4 h after the first injection were measured in serum using the human IFN ELISA kit from PBL Biomedical Laboratories according to the manufacturer’s instructions. This kit has been shown to recognize both unpegylated and pegylated human IFN .
You May Like: Is Hepatitis A Virus Or Bacteria
What Is The Role Of Interferon In The Treatment Of Hepatitis C Virus Infection
Interferon has been the drug of choice for the treatment of hepatitis C for more than two decades. It is often used in combination with another drug, ribavirin. Successful IFN-based therapy, resulting in a sustained virologic response , can improve the natural history of chronic hepatitis C and may reduce the risk of HCC in patients with HCV-induced cirrhosis.
Treatment Of Hcv Genotype 2
The FISSION study compared SOF/RBV for 12 weeks versus peg-IFN/RBV for 24 weeks in treatment-naïve patients and showed an overall SVR rate of 97% for SOF/RBV, whereas peg-IFN/RBV showed only an SVR of 78% in GT2 patients . The POSITRON study showed an SVR > 90% in cirrhotic and non-cirrhotic patients with GT2 who were treated with SOF/RBV for peg-INF intolerance, contraindication, or due to the patients’ decision . Based on these studies, most guidelines consider 12 weeks of SOF plus RBV as the standard of care. Treatment may be extended to 16 weeks in patients with liver cirrhosis.
The combination of the NS5A inhibitor DCV in combination with SOF and RBV for 24 weeks showed an SVR of 92% in GT2 without cirrhosis . This combination can be recommended as a second-line therapy for SOF/RBV relapse patients.
Don’t Miss: Royal Canin Hepatic Wet Food
What Kinds Are There
Scientists have determined that the body makes three distinct types of interferon alpha, beta and gamma interferon, each containing several members. Alpha interferon has been approved for therapeutic use against a specific type of leukemia, hepatitis B and C, genital warts, AIDS- related Kaposis sarcoma and some rare cancers of blood and bone marrow. Nasal sprays containing alpha interferon provide some protection against colds caused by rhinoviruses.
There are two primary types of interferon currently available. To date, interferon-alpha 2a or 2b is the compound that has been extensively used and tested. Though the dose varies, patients with chronic hepatitis C usually receive 3 million units, three times per week. Individuals with chronic hepatitis B receive a higher dose of 10 million units, three times per week. Although it can widely vary, the typical duration of therapy is 48 weeks for hepatitis C, and 16 weeks for hepatitis B.
Side Effects
Interferon used for hepatitis treatment alpha and pegylated forms have been known to cause severe side effects, including:
- worsening of psoriasis
- weight loss
- elevated liver enzymes
- difficulty concentrating and impaired memory
Paritaprevir/r + Ombitasvir + Dasabuvir
In January 2015, the three-target triple therapy 3D consisting of the NS3 protease inhibitor paritaprevir/r boosted with ritonavir , co-formulated with the NS5A-inhibitor OBV , and the non-nucleosidic polymerase inhibitor DSV has been approved for the treatment with or without RBV of chronic GT1 HCV infections. The recommendation is based on six phase III clinical trials with more than 2,300 GT1-infected HCV patients . Approval studies showed that almost every GT1b-infected patient can be cured with this regimen, and also HCV GT1a-infected patients experienced an SVR in more than 95% of cases . Patients with a GT1b infection without cirrhosis can be treated for 12 weeks without RBV while all other patients should receive RBV. It should be noted that no data from phase III trials on the use of this triple therapy regimen without RBV in cirrhotic patients are available at this point in time. Nevertheless, the recommendation for GT1b-infected patients include the combination of OBV/PTV/r + DSV plus RBV for 12 weeks versus a treatment elongation to 24 weeks in GT1a-infected patients with advanced cirrhosis .
You May Like: How Does One Get Hepatitis C