Managing Treatment Side Effects
Connie developed very harsh side effects from the medication. She struggled with extreme fatigue, muscle and joint aches, gastrointestinal symptoms, and hair loss.
Some days were better than others, she recalled, but for the most part, it was severe.
It wouldve been hard to hold down a full-time job, she said. She had worked for years as an emergency medical technician and respiratory therapist. But she had quit shortly before being tested for hepatitis C, with plans to return to school and pursue a nursing degree plans that she shelved after learning she had contracted the infection.
It was hard enough to manage her responsibilities at home while coping with the side effects of treatment. There were days when it was difficult to get out of bed, let alone take care of two kids. Friends and family members stepped in to help with childcare, housework, errands, and other tasks.
ishonestNo.361 – Acne Scars
I was a full-time mom, and I tried to make everything at home as normal as possible for our routine, for our kids, for school, and everything, she recalled, but there were some times I had to have some help.
Fortunately, she didnt have to pay for extra help. We had a lot of gracious friends and family that stepped in to kind of help, so there was no financial cost out for that. I was grateful for that.
About The Hepatitis C Testing Calculator
Hepatitis C Testing Calculator is an interactive tool designed to evaluate the cost-effectiveness of different testing pathways for hepatitis C. The tool uses a mathematical model to simulate HCV disease progression. Users can input custom testing pathways and the cost-effectiveness of each pathway is calculated. This allows users to determine which testing strategies would be appropriate in various localized settings, as hepatitis C prevalence can vary substantially by jurisdiction, and differences in population characteristics and resources create different opportunities for testing and engaging patients in care.
The country can be changed in the tool, which changes the underlying disease burden model. Users can then change the cohort size, HCV prevalence, target screening rate, and treatment cost. An interactive interface allows users to create custom testing pathways, which can include up to 7 different stages: screening, confirmation, liver staging 1, liver staging 2, genotyping, monitoring, and SVR12. The type of test used, cost of the test, testing location , and patient follow-up rate for each stage in the screening pathway can be changed. The total costs and outcomes associated with each testing strategy are displayed, and a preferred testing pathway is recommended based on a cost-effectiveness threshold.
Hepatitis C Treatment: Screening Comes First
Of course, you wont need hep C treatment unless you have the disease. Medicare Part B may cover a one-time hepatitis C screening test if its ordered by your primary care doctor or health-care practitioner.
In some situations, Medicare covers a repeat hepatitis C screening once a year if youre considered high risk for getting the condition. Youre considered high risk for contracting hepatitis C if you meet at least one of the following conditions:
- You were born between 1945 and 1965.
- You had a blood transfusion before 1992.
- You currently use illicit injection drugs, or you have a past history of illicit injection drug use.
Youll pay nothing for this hepatitis C test if your doctor accepts Medicare assignment, meaning that he or she agrees to accept the cost Medicare has approved for the test as full payment and not charge you above that .
Also Check: Latest Treatment Of Hepatitis B
How Much Hepatitis C Medications Actually Cost
Unfortunately, even qualifying for the treatment doesnt mean getting it. With many health insurance plans the deductibles remain ridiculously high.
For example, those who benefit from Medicare health plans have a 50-50 chance of getting their treatment with Viekira Pak® covered. According to GoodRx, the deductible varies anywhere from $7,900 to $32,000, per month.
Technically, almost any Medicare plan will cover hep C treatment with Harvoni®, but the extent of the coverage is undependable. The majority of the plans only cover the treatment for the first 30 days.
In reality it means that your deductible for the first month can be in the $9,000 range, but the treatment for each subsequent month will come with $32,000 bill.
For the reference, recommended treatment duration is either three or six months, depending on previous treatment history with interferon and the exact stage of liver disease. Only in some rare cases the treatment duration can be reduced to two months, but never to one.
What Drugs Cure Hepatitis C Infection
Most hepatitis C is currently treated with all-oral medical regimens of “direct-acting antivirals” or DAAs. DAAs is a term used to distinguish these hepatitis C drugs from an older generation of injected medicines that act indirectly on the immune response to the hepatitis C virus. DAAs act directly on the virus to block different steps in its life cycle. There are several DAAs that are used in combinations that have been scientifically proven to cure hepatitis C. They are not interchangeable, and some are only available combined in one pill or dose pack as a specific combination. DAAs are not used as single-drug therapy because of the high risk of the virus developing resistance and because they work best in combinations. The choice of which regimen to use depends upon the genotype of the virus, the level of liver fibrosis , and any drug resistancethat may be present .
Examples of combination DAAs with cure rates between 91%-100% include:
- Harvoni
- Zepatier
- Mavyret
Genotype 1a and 1b are the commonest genotypes in the United States. Of all the genotypes, genotype 3 has been the most difficult to treat with DAAs alone and required the use of ribavirin, which has significant side effects. All genotypes can now be treated with oral DAAs without ribavirin. Some genotypes may still require the use of injected pegylated interferon and/or ribavirin if there is no response to DAAs.
Read Also: How To Cure Hepatitis A Fast
Hep C Treatment Refused To All But Critical Patients
In 2014 in California, for example, 94% of those who requested hepatitis C drugs through Medi-Cal were denied coverage. Meanwhile, in the same fiscal year the state has paid $58 million to treat prisoners with advanced stages of hepatitis C.
The situation in California is not unique. Of the 42 states where Medicaid actually reimbursed sofosbuvir, the active substance of Sovaldi® and Harvoni®, 74% limited treatment access only to persons with advanced fibrosis or cirrhosis .
The vast majority of states include drug or alcohol use in the eligibility criteria, requiring either a period of abstinence or urine screening two third of states impose restrictions based on prescriber type.
Medicaid reimbursement criteria for sofosbuvir based on liver fibrosis stage Annals of Internal Medicine
Among the commonly recommended treatment regimes for hepatitis C are Sovaldi®, Harvoni®, Epclusa® and Viekira Pak®.
These limitations dont match the latest recommendations for hepatitis C treatment. In fact, initiating therapy in patients who did not yet develop severe fibrosis augments treatment benefits, while treatment delay decreases it.
Treatment is recommended for all patients with chronic HCV infection, except those with short life expectancies that cannot be remediated by treating HCV, by transplantation, or by other directed therapy. HCV Guidelines
Simply put, the insurers are caught between Big Pharmas sky-high prices and HCV advocates who say that rationing care is illegal.
Treatment Regimens And Efficacy
We simulated two strategies: no treatment and treatment with available DAAs. The DAA treatment regimens used were determined by individual patients HCV genotype and METAVIR fibrosis stage. The treatment efficacy in various scenarios was based on the SVR rate reported in clinical trials of DAAs, and uncertainty in SVR rates was incorporated in sensitivity analysis. Regimen-specific treatment discontinuation rates were also incorporated in the model. Data about the treatment regimens were obtained from recent clinical trials of DAAs in treatment-naïve patients,,, and are provided in Supplementary Table .
Recommended Reading: How Many Hepatitis B Shots Are Required
Evaluation Of Individuals With Positive Screening Test
Patients with a positive screening test for anti-HCV antibody should be tested for serum HCV RNA. Serum HCV RNA quantifies the amount of viral RNA in serum and indicates ongoing infection. If HCV RNA is detectable, tests should be performed to determine the extent of hepatic fibrosis. These tests typically include liver biopsy or noninvasive measures, such as biochemical markers of fibrosis or transient elastography., An ultrasound of the abdomen should also be performed to identify the possible presence of cirrhosis and focal lesions in the liver suspicious for hepatic malignancy.
Patients who have a positive anti-HCV on a screening test but have no detectable HCV RNA should have a confirmatory HCV RNA test a few months later. If HCV RNA remains undetectable, these individuals should be reassured that they do not have hepatitis C infection and that the anti-HCV may remain persistently positive. Such individuals have either cleared the virus or the true specificity of the test is lower than the reported 100%, and the test result was a false positive.
When Private Insurance Isnt Better
Even with a reasonably good private insurance the modern hepatitis C drugs remain out of reach for most. Private insurance companies deny coverage just as often as Medicare, arguing that the patients are not sick enough.
And theres the same clash between existing recommendations: insurance guidelines allow treating the patients with at least fibrosis level F3, while official HCV guidelines recommend that all the patients, except those with short life expectancies, get treated as soon as possible.
So what should you do if you dont qualify or cant afford the treatment? There are plenty of things you could try, and were not talking about trying outdated treatment regimens, starting a crowdfunding campaign or robbing a bank. Heres a detailed overview of available solutions.
In summary, the most important thing is to seek assistance everywhere and not to give up. Many non-profits and pharmaceutical companies have hepatitis C support programs that help to pay the deductible.
Waiting to develop advanced liver disease in order to be covered is utterly frustrating. Worse even, if you wait too long or try too many cheaper and less effective treatments, the new antivirals might simply not work for you!
Read Also: What Is Hepatic Steatosis Mean
Hep C Treatment Yes But Not For Everyone
Another problem is, the miraculous Harvoni® doesnt work its magic for all the genotypes. Until recently, patients with genotypes 2 and 3 had to use Sovaldi® in combination with either interferon or Daklinza®.
A three-months-long treatment with Daklinza® comes with $61,000 price tag. As for Sovaldi®, the out-of-pocket costs are around $80,000, and starting from 2016 the drug is no longer covered at all.
On the bright side, later this year FDA approved a new drug for hepatitis C. Epclusa® can be successfully used for hepatitis C treatment regardless of genotype and when it comes to genotypes 2 and 3, its efficiency is slightly superior compared to the previously existing regimens. It is cheaper, too: starting at only $71,328 per three bottles.
Whats the bad news? Medicare wouldnt pay for it.
Who Can Help Me
If youre concerned about paying for HCV medications, remember that you arent alone as you seek treatment. There are people and organizations that can help you, including the following:
- Your doctor. They can help you by ordering and documenting the tests youll need so you can qualify to get your medications, especially if youre working with a liver or infection specialist.
- Most drug manufacturers. There are patient assistance programs that offer free or reduced-cost medications for people who meet their criteria.
- Patient advocacy groups. These groups provide assistance with all aspects of HCV treatment. For instance, if your insurer denies treatment, you can appeal the decision with help from one of these groups. Your doctor can also help in this situation.
Drug companies and patient advocacy groups are a great place to start when looking for help paying for HCV medications. Heres a list to get you started.
Read Also: How Much Is Hepatitis A Vaccine
Prices Costs And Affordability Of New Medicines For Hepatitis C In 30 Countries: An Economic Analysis
-
Affiliation World Health Organization, Geneva, Switzerland
-
Affiliation WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH, Vienna, Austria
-
Affiliation World Health Organization, Geneva, Switzerland
-
Affiliation World Health Organization, Geneva, Switzerland
-
Affiliation World Health Organization, Geneva, Switzerland
-
Affiliation World Health Organization, Geneva, Switzerland
The Real Cost Of Hepatitis C Medications
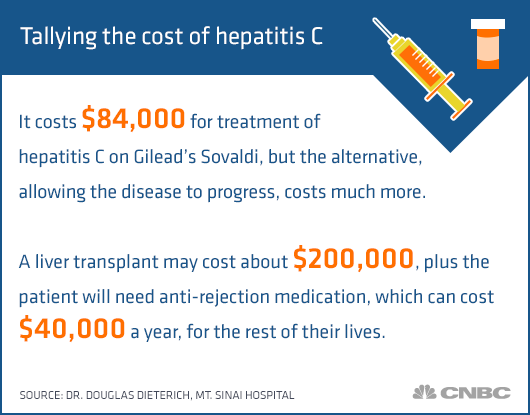
New hepatitis C drugs are extremely effective, but their price tags are a jaw-dropper. A three-month treatment course can easily cost $70,000-$100,000. Its pretty obvious that at this price the vast majority of uninsured patients cant afford the treatment. What is little known though, is that the situation is hardly better for those covered by state insurance programs.
Recommended Reading: Royal Canin Hepatic Wet Food
Characteristics Of Base Case Population
We simulated a total of 150 unique HCV-patient profiles based on five age categories , three HCV genotypes that are prevalent in Japan , patients sex and METAVIR fibrosis score . The distributions of these HCV-infected patient profiles were estimated based on available data . The model did not include patients with HIV or hepatitis B virus co-infection as well as special groups at higher risk of HCV reinfection, such as those with hemodialysis, thalassemia, haemophilia or injection drug use. All patients were considered treatment-naïve because the current percentage of treatment-experienced patients in Japan is small.
Drawbacks To Screening For Hepatitis C
A patient’s worry or anxiety while waiting for test results, concerns about insurance coverage of evaluation and treatment, adverse effects of treatment, and complications associated with liver biopsy can limit screening for hepatitis C. However, the accuracy of HCV RNA testing, the availability of medication assistance programs for uninsured patients, and improved treatments with fewer side effects and shorter duration should allay some of the anxiety associated with the screening process.
Also Check: Where Do I Get Hepatitis A Vaccine
Hepatitis C Treatment: Medicare Coverage Of Liver Transplants
In severe cases, your doctor might recommend a liver transplant might be recommended for hepatitis C patients. In fact, according to the Centers for Disease Control, hepatitis C is the most common reason for liver transplant surgery in the United States.
If this is medically necessary for your hep C treatment, Medicare Part A generally covers the hospital portion of the procedure under certain conditions at an approved, Medicare-certified facility, while Medicare Part B may cover any doctor services needed as part of your liver transplant.
Youll typically be responsible for paying 20% of the Medicare-approved amount for doctor services for hepatitis C treatment, and the Part B deductible applies. Medicare generally covers your lab tests as part of your hepatitis C treatment. You may need to pay various out-of-pocket costs- for example, for doctor visits and transplant facility charges.
I hope you now have a better understanding of how Medicare covers hepatitis C treatment and what your options may be. Are you looking for more coverage to help with your hepatitis C treatment or management? I can help you find a or a Medicare Advantage Prescription Drug Plan that may fit your health needs and your budget.
You can click one of the links below to set up a time to talk by phone or get a personalized email with plan information. Compare the plan options in your location by clicking the Compare Plans or Find Plans buttons on this page.
New To Medicare?
How Part D Plans’ Preference For Higher Cost Hepatitis C Drugs Affects Medicare Beneficiaries
In 2019, Medicare Part D spent approximately $2.5 billion for hepatitis C drugs to treat 50,000 beneficiaries with the disease. Three drugsHarvoni, Epclusa, and Mavyretaccounted for 93 percent of expenditures, with annual Medicare costs ranging from $28,000 to $77,000 per beneficiary. A portion of these totals was shared by Medicare beneficiaries who faced thousands of dollars in out-of-pocket costs for hepatitis C drugs under Part D. In early 2019, Gileadthe manufacturer of Harvoni and Epclusalaunched authorized generic versions of both drugs with the expressed goal of reducing patients’ outofpocket costs. The retail price of authorized generic versions is $24,000, which is significantly less than the prices of Harvoni and Epclusa, and even less than Mavyret. These lower list prices should in turn lead to lower out-of-pocket costs, as authorized generics are as effective as branded versions but sell for only a fraction of the cost. However, a preliminary analysis indicates that Medicare utilization has not shifted from brandname versions of Harvoni and Epclusa to their significantly cheaper, authorized generic versions or to Mavyret. This study will examine the utilization of hepatitis C drugs under Part D and the financial impact on Medicare Part D and beneficiaries.
| Announced or Revised |
|---|
Read Also: Can Hepatitis Cause Low Platelets
Hepatitis C Treatment Cost In India
Hepatology
Hepatitis C Treatment Cost in India
Hepatitis C is a viral disease that attacks the liver and causes inflammation. Approximately 71 million people throughout the world are estimated to become infected by HCV. Eastern Mediterranean and European areas are the most affected areas with 2.3% and 1.5percent of the populace experiencing HCV respectively. HCV doesnt lead to symptoms normally consequently, infected individuals dont come to understand for long. The HCV disease causes about 400 million deaths each year, mostly because of chronic hepatitis C resulting in cirrhosis or hepatocellular carcinoma.
The Real Cost Of Hep C Medications
When it comes to modern hepatitis C drugs, the patent owners argue that despite their high prices it is still a fair deal. And it is, if we compare these antivirals to their only alternative liver transplant that can easily cost $250,000 and more.
Everyone knows that the market price of these drugs is set according to its perceived value and has nothing to do with their production cost. However, very few of us know how much the wonder drugs cost to produce.
Dr. Andrew Hill and his team from the University of Liverpool have recently performed a study about large-scale manufacturing of modern hepatitis C antivirals. The results are shocking, to say the least. The minimum manufacturing costs of antivirals were estimated at $0.2$2.1 per gram. For instance, a 12-week course with daclatasvir and sofosbuvir would costs less than $200 dollars to produce. Put the exact same active ingredient in branded packaging , and it is sold at $141,000. This actually means that the profit margins for hepatitis C meds are way higher than those of illegal drugs!
Manufacturing costs of hepatitis C meds were estimated at $0.2$2.1 per gram.Thats less than $200 per treatment course.
Dr. Hills study also explains why it makes perfect sense for Big Pharma to sign license agreements for generic production, like the ones signed with Indian pharmaceutical companies in 2015.
Also Check: How Is Hepatitis C Spread