Hbeag Positive Hbv Carriers
HBeAg is not essential for virus replication. HBeAg negative variants of HBV with a mutated, non-functional preC sequence were first described in 1989 by William Carman and can even cause fulminant hepatitis B. As shown by David Milich, HBeAg acts as an immune modulator and suppresses the recognition of HBcAg expressing cells by T-lymphocytes which is the main mechanism of HBV immune control. The long term lack of effective immune defenses allows production and secretion of the virus with up to 1010 infectious particles per mL blood, without the infected person showing clinical symptoms. HBsAg is usually present at very high levels of between 30,000 and 200,000ng/mL serum .
The three phases of chronic HBV infections.
Cd81 Is A Target Of Positive Selection In Bats
Host receptors that mediate virus entry often evolve under positive selection to avoid viral recognition .
Four cell-surface molecules, CD81, occludin , claudin 1 , and scavenger receptor class B member 1 , are particularly important for HCV cell entry, although only CD81 and OCLN determine the species-specificity of infection . We thus reasoned that if primates experienced long-term interactions with HCV or closely related hepaciviruses, signals of positive selection should be detectable at the genes encoding HCV receptors. Conversely, if selection is evident in other mammalian orders, these may represent the ancestral HCV reservoirs.
We retrieved coding sequences of the four genes for mammalian species belonging to different orders, superorders or clades . Due to the hypothesized role of bats as reservoir hosts for mammalian hepaciviruses, Chiroptera were analyzed separately from other species in the Laurasiatheria superorder. Evidence of positive selection was searched for using models that allow dN/dS to vary among sites in the alignment .
No evidence of positive selection was detected for CLDN1 and SRB1 . Also, selection was not detected at OCLN or CD81 in primates , an observation that does not support a long-standing selective pressure exerted by hepaciviruses on these hosts. However, positive selection was not detected for rodents, either, although these mammals are known to host a wide diversity of hepaciviruses .
Discovery Of The Dane Particle
AuAg, however, was not a prion-like agent. While inspecting AuAg immune complexes under the EM in 1970, David S. Dane discovered that AuAg appeared not only on the small pleomorphic particles, but also on larger, virus-like objects 42nm in size with a clearly visible inner core . Shortly thereafter, in 1971, his British colleague June Almeida was able to release the core particles from the so-called Dane particles by treatment with mild detergent, and showed by immune EM that hepatitis B patients formed antibodies against this core antigen . This strongly suggested that the Dane particles were the actual virus causing hepatitis B. AuAg was obviously the surface antigen of the virus envelope, and was named HBsAg thereafter. The infected hepatocyte forms the HBsAg protein in large surplus and secretes it in addition to the complete virus as round or filamentous noninfectious particles of about 20nm in diameter into the blood leading to an approximately three-thousand fold excess of these subviral particles .2). This was the reason that the Dane particles could not be recognized in AuAg preparations purified by ultracentrifugation or size chromatography.
Read Also: How To Screen For Hepatitis C
The Bottom Line: Anyone Can Get Hep C
Heres the thing: Its a myth that only bad or irresponsible people get hepatitis C, says Dr. Goff. While its true that certain behaviors can increase your risk, it helps to remember that many reasons why people get hepatitis C were out of their control. Its not your fault that you got this viral infectionlife simply happens. People from all walks of life have hepatitis C, Dr. Fox reminds us. The important thing is to decide how youll best take care of your health moving forwardlikely starting with effective hepatitis C treatment.
- World Health Organization Hepatitis C Facts: World Health Organization. . Hepatitis C. who.int/news-room/fact-sheets/detail/hepatitis-c
- Hepatitis C Information From the CDC: The Centers for Disease Control and Prevention. . Hepatitis C Questions and Answers for the Public. cdc.gov/hepatitis/hcv/cfaq.htm
- Tattoo and Piercing Safety: The Mayo Clinic. . Tattoos: Understand risks and precautions. mayoclinic.org/healthy-lifestyle/adult-health/in-depth/tattoos-and-piercings/art-20045067
- Hepatitis C in Pregnancy Guidelines: Society for Maternal-Fetal Medicine. . Hepatitis C in pregnancy: screening, treatment, and management. ajog.org/article/S0002-937830930-4/pdf
- Kidney Disease and Hep C: The National Kidney Foundation. . Hepatitis C and Chronic Kidney Disease: Overview of Evaluation and Management. kidney.org/atoz/content/hepatitis-c-and-chronic-kidney-disease-overview-evaluation-and-management
Hep C Is A Bloodborne Disease
It can be distressing to learn you have hep C, especially if you dont know how or when you were infectedand many people dont, says John Goff, M.D., clinical professor of medicine at the University of Colorado School of Medicine and member of the American Liver Foundations National Medical Advisory Committee. It helps to learn how hep C can even spread in the first place: It is transmitted primarily through blood-to-blood contact, says Rena Fox, M.D., an internist and hepatitis specialist at UCSF Health and a professor of general internal medicine at UC San Francisco. Lets go over some of the risk factors you may already know aboutplus, a few surprising ones.
Don’t Miss: What Does Immunity To Hepatitis B Mean
Detection Of Antiviral Antibodies
As an alternative approach, detection of antibodies which the patients had produced against the antigens of the disease-causing agents was adopted as a diagnostic method. However, this approach was problematic as it depended upon detection of the reaction of the patients antibodies with the viral antigen. The method most often used was the quite difficult complement fixation reaction , which was originally developed for diagnosing syphilis. In addition to the human patient serum, and the viral antigen , one needs sheep erythrocytes as indicator cells, rabbit antibodies against the sheep erythrocytes for generating an immune complex on the erythrocyte membrane and, finally, complement for the CFR. Complement is a multi-protein complex in animal sera that binds to immune complexes. When assembled and activated on cell membranes, holes are punched in the cells by the complement, which leads to lysis of the cells. If the patient serum does not contain antibodies, the complement lyses the erythrocytes, and the non-transparent red reaction mix becomes a transparent red. If immune complexes were formed previously in the mix of patient serum and viral antigen, these bind the complement away, such that it no longer can lyse the erythrocytes. CFR requires four complex biological component mixtures from four different animal species and these mixtures must all be standardized quantitatively by the individual lab.
Waning Hbeag And Chronic Hepatitis B
In patients with chronic hepatitis B, the immune defense is partially active, such that the viral loads and HBsAg levels are lower . While the destruction of the HBV-infected liver cells leads to chronic liver inflammation, it does not stop the infection, as new cells are continuously infected in the absence of neutralizing antibodies. Only once the immune defenses become more efficient can the patient reach a condition where HBsAg is still produced, but the production of virus particles is so slight , that it can no longer cause any major damage. These quasi-healthy HBsAg carriers have less HBsAg in the blood and no longer have HBeAg, but rather the corresponding antibody, anti-HBe. A seroconversion of HBeAg to anti-HBe and a significant decrease of HBsAg are considered a good sign for a spontaneous or therapy-induced improvement. But many patients who have lost HBeAg may still suffer from progression of chronic hepatitis B because the virus has an enormous capability to evade T and B cell immunity even if HBeAg is no longer present.
Read Also: How Long Does A Person Live With Hepatitis C
Chemical Labeling Of Antibodies
In 1972, a team including biochemists Lacy Overby, Ghung-Mei Ling and Richard Decker at Abbott Laboratories developed a new testing principle for highly-sensitive detection of antigens or antibodies, the solid-phase sandwich radioimmunoassay named Ausria 125. The test combined the specificity of the biological antigen-antibody interaction with the high sensitivity of modern physicochemical analytical methods. Proteins can be covalently coupled with many physically detectable components. Even before 1972, it was possible to couple e.g. fluorescent molecules to antibodies. Antibodies visible under UV light were specifically used in the recognition of viral and other causal agents in microscopic tissue preparations , and conversely fluorescently marked animal antibodies against human antibodies could be used to determine whether a patient serum contained antibodies against a pathogen-specific antibody under the microscope. For viral hepatitis, however, there were no susceptible tissue cultures. One part of this new test took up the newly developed technique of labeling antigens or antibodies with radioactive substances such as iodine-125 which had already facilitated e.g. the detection of insulin and other small molecules by biophysical techniques.
Association With Membranes And Lipids
All HCV proteins are tethered to intracellular membranes either via transmembrane domain or through amphipathic alpha helices or both . We identified several positively selected sites within amphipathic alpha helices . In the case of NS4B, virtually all sites are located in these regions, where several RAVs also map . Among these, W43 is essential for NS4B association to the membrane and to lipid droplets .
We found positively selected sites within the sphingomyelin binding domain of the HCV RNA polymerase . Binding of sphingomyelin to NS5B allows localization of the polymerase to lipid rafts and activates the enzymatic activity in a genotype-dependent manner . Remarkably, mutagenesis experiments indicated that the positively selected sites 244 and 238 modulate sphingomyelin binding and activation, respectively . We thus docked a sphingomyelin molecule onto the 3D structure of subtype 1b NS5B . Docking results confirmed a salt bridge interaction between residue 244 and the sphingomyelin and the localization of residue 238 at the interaction surface. Moreover, analysis of atomic distances indicated that two additional selected sites are likely involved in the binding of sphingomyelin .
Also Check: Is Hepatitis C Considered An Std
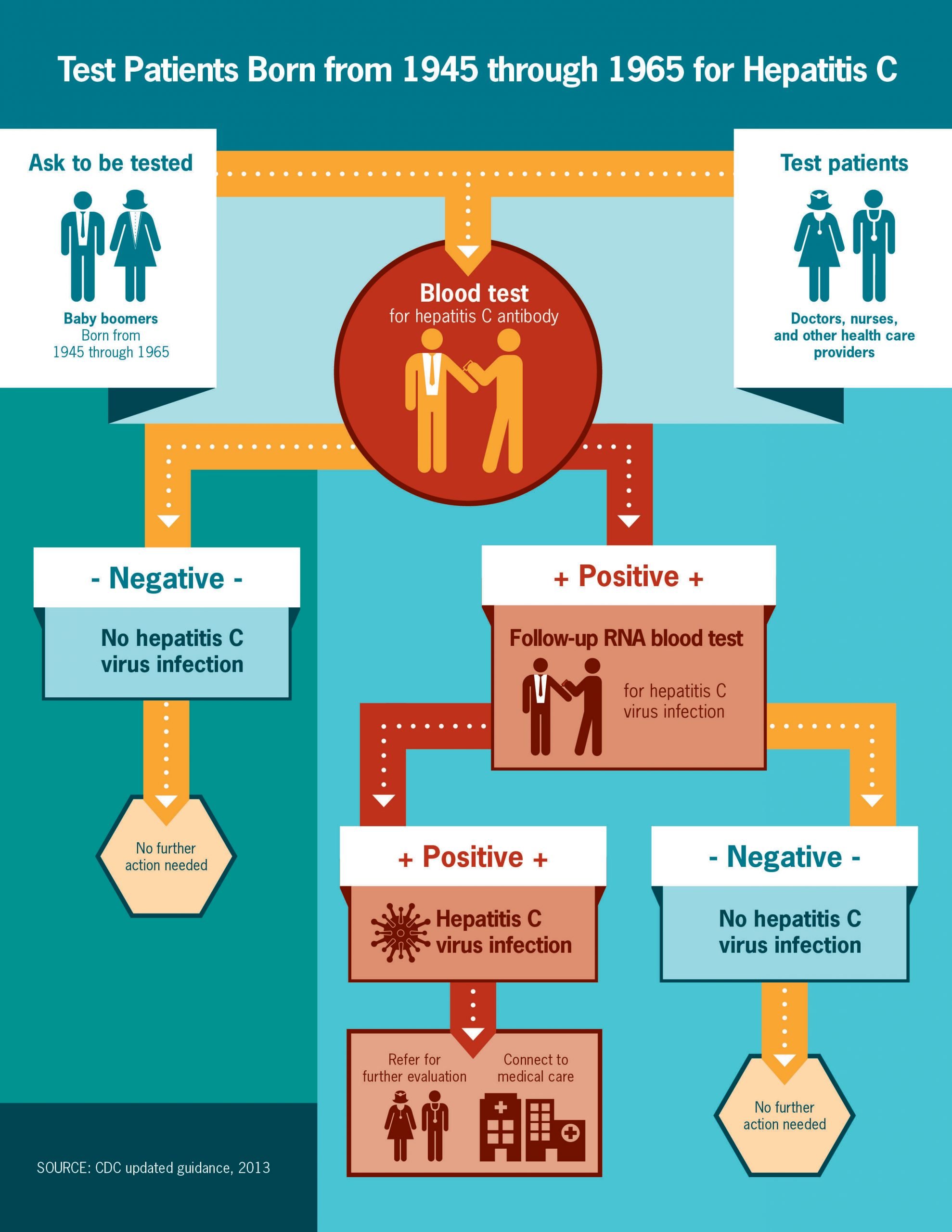
Tests To Diagnose Hepatitis C
How is Hepatitis C diagnosed?
There are two main blood tests typically used to diagnose Hepatitis C. First, youll have a screening test that shows if youve ever had Hepatitis C at some point in your life. If this test is positive, youll have a second test to see if you have Hepatitis C now. These blood tests are described below:
Hepatitis C antibody test
This is the screening test used by doctors to show whether or not you have ever been exposed to Hepatitis C at some time in your life, by detecting antibodies in your blood. Antibodies are substances your body makes to fight off all kinds of infections. If you were ever infected with Hepatitis C, your body would have made antibodies to fight the virus.
If the test result is:
- Negative, it means you have not been exposed to Hepatitis C and further testing is usually not needed.
- Positive, you have had Hepatitis C at some point. However, it does not tell you whether you have it now. Youll need to see your doctor for another test the Hepatitis C RNA test to determine if the virus is still active and present in your blood.
Hepatitis C RNA Qualitative Test
This test will determine whether or not you are currently infected with Hepatitis C. It is often called the PCR test because of the process used . It looks for the genetic material of the Hepatitis C virus in your blood.
If the test result is:
Hepatitis C RNA Quantitative Test
Zeroing In On The Hepatitis C Virus
The era of direct-acting antivirals that specifically target HCV began in 2011 with the U.S. Food and Drug Administration approval of the first protease inhibitors. These drugstelaprevir and boceprevir, along with several similar drugs approved latertargeted the HCV protease that is critical for viral replication. When used in conjunction with peginterferon and ribavirin, protease inhibitors yielded SVR rates of up to 75 percent. However, this triple therapy was accompanied by additional side effects to those already present with peginterferon and ribavirin. Nevertheless, the success of HCV-specific protease inhibitors showed that the virus had vulnerabilities that could be exploited by a well-designed and properly administered drug.
More new anti-HCV drugs were developed and tested over the next several years. These new drugs included sofosbuvir and dasabuvir, which interfered with the activity of the HCV polymerase, an enzyme that is responsible for the viral replication. Members of a second class of drugs, ledipasvir and daclatasvir, targeted the NS5A region of the virus, which makes a structural protein critical for viral replication. Many of these drugs were initially tested in conjunction with peginterferon and ribavirin, or in combination with a protease inhibitor. Generally, the results were SVR rates of at least 80 percent.
Don’t Miss: Hepatitis B Core Ab Total Reactive
How Do People Get Hepatitis C
Hepatitis C virus is found in the blood of people with HCV infection. It enters the body through blood-to-blood contact.
Until reliable blood tests for HCV were developed , people usually got hepatitis C from blood products and blood transfusions. Now that blood and blood products are tested for HCV, this is no longer the typical means of infection.
Currently, people usually get hepatitis C by sharing needles for injection drug use. An HCV-infected woman can pass the infection to her baby during birth. It is also possible to get hepatitis C from an infected person through sexual contact, an accidental needlestick with a contaminated needle, or improperly sterilized medical, acupuncture, piercing, or tattooing equipment.
Indication For Interferon Therapy

After 36 years of experience, interferon alpha still has its place in HBV therapy, but the patients need to be carefully selected, because interferon has many severe side effects and contra-indications, and only a minority will show a sustained response. Interferon suppresses HBV replication but the exact mechanism is not known and today more dependable chemical antivirals with less side effects are available. The main advantage of interferon is that it can enhance the bodys own immune defense, and accelerate the sustained resolution of the infection. Thus, patients with active inflammation, i.e. elevated transaminsases, and partially successful immune control are the best candidates for this strenuous therapy. The cccDNA form of the HBV genome is probably as stable as the host chromosome and can currently not be attacked by any available drug. However, interferon may induce innate defense mechanisms e.g. the RNA editing cytidine deaminase APOBEC3G, which may damage the HBV pregenome, or it may enhance apoptosis of infected cells. Accelerated hepatic cell turnover in absence of HBV replication will decrease HBV cccDNA to innocuous levels. Long-term follow-up has confirmed that patients with a sustained viral response regain a normal life expectancy .
Recommended Reading: What Are Some Symptoms Of Hepatitis C
Mutation Rate Of The Hepadnavirus Genome
An essential factor for calculating the evolutionary divergence of hepadnaviruses is the inferred mutation rate of the virus genome. It is defined as the number of base substitutions within the genome per site per year of continuous virus replication in the host. HBV mutation rates are often inferred by comparing the HBV sequences either from mothers and children of maternally acquired cases, or chronic carriers over a specific time frame. However, these mutation rates are considered short term, and should be used with caution. There are two important observations to support this claim. First, mutation rates can vary by at least one order of magnitude if the HBV genome sequences used for inferencing were from individuals in the HBeAg-negative phase of disease instead of being in the asymptomatic HBeAg-positive phase . Second, HBV mutations have the tendency to revert back to the genotype consensus over time , thus, suggesting that the HBV genome would not have changed significantly over time despite having a high mutation rate . These observations further support the model that HBV has a long evolutionary history and may have coevolved with humans in the ancient past.
Where Did Hepatitis B Virus Come From
| Font : A-A+ |
New insight on the geographical origins and the global spread of two classes of the hepatitis B virus has been found in this study. The findings of this study are published in the journal of eLife. The findings identify the HBV genotypes D and A as having originated in the Middle East and North Africa. They also reveal considerable differences in the global dissemination patterns of these genotypes, adding to our understanding of both the historical and prehistoric spread of one of the world’s largest viral pandemics.
Recommended Reading: Can You Cure Hepatitis A
Hcv Originated At Least 3000 Years Ago
Previous studies provided estimates of the time to the most recent common ancestor of HCV genotypes in a range between 200 and 1000 years ago, with one single study indicating that HCV origin may date 2000 years back . The tMRCA of equine/canine hepaciviruses was estimated to be recent, dating around 1800 CE .
It is well known that the temporal variation in rates of nucleotide substitutions often results in underestimation of the age of viral lineages . Purifying selection and substitution saturation are strongly associated with temporal rate variation .
Simulations experiments indicated that classic models tend to underestimate branch lengths in the presence of purifying selection and substitution saturation . Because both phenomena are more pronounced for internal branches, length underestimation is more severe for these branches and dating inferences are consequently affected . The use of models that allow site- and branch-specific variation in selective pressure can improve branch length estimates in the presence of both purifying selection and substitution saturation .
FIGURE 2. tMRCA estimation. Comparison of branch lengths obtained using the aBS-REL and the GTR models for the NS5B abd EHV phylogenies. Timescaled phylogenetic tree estimated for 67 HCV subtypes. The scale bar below the phylogeny represents years before present. The tMRCAs of analyzed nodes are reported in red with 95% confidence intervals. Geographic distribution of HCV endemic transmissions .