Validation Of The Assay With International Standards
For validation of the assay, the international panel cited above was used in qPCR reactions to construct standard curves of HBV DNA or HCV RNA in the following concentrations: HBV 7 or HCV 7 , HCV 6 or HBV 6 , HBV 5 or HCV 5 , HBV 4 or HCV 4 , HBV 3 or HCV 3 , and HBV 2 or HCV 2 . The reactions were performed in a 7500 Real-Time PCR System using the TaqMan detection system with 12.5 µL of TaqMan Universal Master Buffer, predetermined concentrations of the primer-probe sets cited above, and 50 – 100ng of DNA or cDNA, for a total final volume of 25 µL per reaction. All reactions were performed in duplicate using universal conditions: 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. Results were analyzed using 7500 Fast Software v. 2.1 and expressed in IU/mL. The baseline and threshold values were automatically adjusted for each test.
Preparation Of Duplex Mutation Primers
The fluorophore primers and the reverse primers were used to amplify a 94-bp fragment in the highly conservative 5′ non-coding region of the HCV RNA. The fluorophore primers were quenched by partly complementary oligonucleotides of a single-base mismatched labeled with a quencher at 3′-end. All of the primers were selected using the primer premier 5.0 software and were synthesized, purified and labeled by Takara Ltd. Dalian, China.
Extraction Of Nucleic Acids
Nucleic acids were extracted from 200 µL of serum using a QIAamp MinElute Virus Spin kit according to the manufacturer’s suggested protocol. The concentration and quality of the extracted DNA and RNA were assessed by Nanovue spectrophotometry and by amplification of a fragment of the gene coding for β-actin . The extracted DNA and RNA were stored at -80 °C until use.
You May Like: Hepatitis C And Liver Failure
We Implement Proven Measures To Keep Your Data Safe
At HealthMatters, we’re committed to maintaining the security and confidentiality of your personal information. We’ve put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
Popular search
Validation Of The Assay With Serum Samples
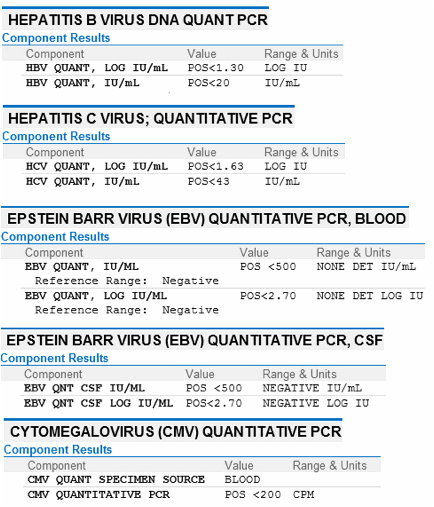
To validate this assay, serum samples were collected from patients infected, either acutely or chronically, with HBV or HCV and maintained at -20 °C until use. The inclusion criterion was the presence of HBV or HCV infection. The exclusion criterion was the absence of HBV or HCV infection. This set of 49 HBV and 67 HCV positive sera were used to validate the qPCR assay. For the qPCR reactions, a dilution series of the standard plasmid was used as the standard curve.
Also Check: Is There A Treatment For Hepatitis C
Reproducibility And Specificity Of The Real
In order to determine the reproducibility of intra- and inter-measurements of the qPCR, serial dilutions were tested on 2 separated days with triplicates of each dilution in each run . shows the mean CV of Ct values and the input copy number of HCV within a run the CV calculated for these repetitions was less than 1.06%. Interassay CV was low for standard curve and samples , a reasonable target for the percentage of CV in routine testing is 10%15%. On the other hand, the reproducibility of the RT-PCR was determined using 4 samples from patients chronically infected with HCV genotypes 1, 2, and 3 the most frequent genotypes in Northeast Mexico.20,23 shows detection of all HCV subtypes tested. The CV of these assays was less than 0.45%, and each sample was tested in triplicate.
The specificity of the RT-PCR we developed was assessed by testing serum/plasma samples from 5 healthy blood donors. All of these samples were negative for HCV-RNA.
Viruses For Analytical Studies
The Chinese National standard for HCV, which has an assigned concentration of 1.25Ã10 IU/mL, was serially diluted in negative human plasma to a final concentration ranging from 1Ã10 to 1Ã10 IU/mL, and were used as reference standards for each real-time PCR run. Analytical verification studies were performed with a commercial panel of clinical specimens containing HCV Gt 1a, Gt 2b, and Gt 3a viruses . For a standardized evaluation, we obtained a Chinese National reference viral quality control plasma preparation panel containing well-characterized HCV RNA levels. These samples were tested extensively and contain HCV RNA levels ranging from no HCV RNA to 107 HCV molecules per mL.
Don’t Miss: Can You Get Rid Of Hepatitis B
Validation Of The Qpcr Assay With International Standards
To validate the assay for quantitation of HBV and HCV, a standard curve was constructed using HBV DNA or HCV RNA from an international panel. The amplification plots and standard curves of both viruses are shown in Fig. 2. Regressions of the Ct values and concentrations of HBV DNA or HCV cDNA showed correlation coefficients of 0.983 and 0.963 for HBV and HCV, respectively. The slopes of the standard curves for HBV and HCV were -3.438 and -2.898, respectively.
Fig. 2
What Your Hepatitis C Virus Quantitative Real
When your results show a high viral load, this normally indicates that the infection level is higher. However, for hepatitis C, a high viral load is not related to how you feel and how damaged your liver is. This number is the perfect indicator of how well a treatment is being successful.
If your viral load is less than 615 international units per liter then, your system does not contain any detectable hepatitis C virus. This result could even indicate that the levels are too low to be detected.
If the viral load is higher than 800,000 international units per liter means that the viral count is still high and the treatment needs to be adjusted. If the levels are below that number this indicates a successful treatment. After a period of eight to twelve weeks, if the viral load became undetectable, this marks the end of the treatment. You will no longer need to be treated for hepatitis C but, still need to be tested after a while to check for a relapse.
Read Also: What Is The Difference Between Hepatitis B And C
Hepatitis C Viral Rna Quantitative Real
Quest Price: $344.49
Hepatitis C is a liver disease that is caused by the hepatitis C virus . Hepatitis C is a bloodborne disease and exposure to the blood of an infected person may result in infection. This test is used to detect and confirm hepatitis C virus infection. Abnormal results may require further evaluation by your physician.
Includes:
Data Acquisition And Analysis
A PCR amplification curve for each amplicon was generated by plotting the peak height versus the cycle number based on the growth of the target peak as the PCR progressed. The quantitative cycle values were determined from the amplification curves using a threshold peak height of 5. Cq values were then converted to IU/mL based on a predetermined calibration curve traceable to the 5th WHO International Standard for HCV. The analysis were conducted using a proprietary software developed for the TASWako g1 instrument.
Recommended Reading: Can You Get Hepatitis C From Your Own Blood
Hepatitis C Antibody With Reflex To Hcv Rna Quantitative Real
Test Code: 8472
Methodology: Immunoassay
Includes: If Hepatitis C Antibody is reactive, then Hepatitis C Viral RNA, Quantitative, Real-Time PCR will be performed at an additional charge.
Clinical Significance: Hepatitis C Virus is a major cause of hepatitis. The clinical symptoms of an HCV infection are variable. Infection with HCV results in a chronic infection in 50 to 80% of cases. The “window” between HCV acquisition and seroreactivity is highly variable up to six months.
Alternative Name: HCV with Reflex,HCV Antibody,Anti HCV
Supply: T01 – Red/Gray SST 8.5mL
Preferred Specimen: Serum
Transport Container: Serum Separator Tube
Transport Temperature: Room Temperature
Specimen Stability: Room Temperature: 72 hours
Rejection Criteria: Moderate Hemolysis, Gross Lipemia
For additional supply or collection device information, please contact DLO’s Customer Service at 891-2917, option 2.
What To Expect During Testing

A healthcare provider will take a blood sample for analysis.
Before the test, let them know if youre uncomfortable with certain needles or if youve ever passed out at the sight of blood. They can give you a snack to reduce your risk of fainting.
The needle may sting a little as it enters your skin, and you may have a bruise on the site of the draw for a few days.
Results are usually available within a few days or a few weeks at most.
The HCV RNA PCR test is conducted through a process called polymerase chain reaction . There are two approaches to this process: qualitative and quantitative.
Also Check: Do I Have Hepatitis C
Optimal Concentration Of Ic
Armored RNA was serially diluted and then spiked into the national reference material for HCV RNA . Armored RNA was coextracted and coamplified with the samples in the same reaction tube. According to the results presented in Table , 1000 copies/ml of armored RNA was used as the optimal concentration of IC in the HCV RNA duplex real-time RT-PCR assay.
Table 1 Optimization of the concentration of IC
Selection And Design Of Primers And Probes For Quantitative Real
The sequences of the primer and probes used in this study are listed in Table 1. The primers were selected from published literature, and the probes were designed using Primer Express Software . Programs, including AmplifX , were used to predict the behavior of these primers. The expected hybridization and specificity of primer-probe sets targeting HBV DNA and HCV RNA were determined by in silico analysis using the BLAST program . The reporter dye FAM was attached to the 5′ ends of probes, and a nonfluorescent quencher and minor groove binder were attached to the 3′ ends. All primers and probes were synthesized by Integrated DNA Technologies.
Read Also: What Does Hepatitis Feel Like
What Is The Role Of Quantitative Hepatitis C Virus Rna Assays In The Diagnosis Of Hepatitis C Infection
Quantitative assays ascertain HCV RNA quantity in blood, using signal amplification or target amplification techniques . RT-PCR is more sensitive than bDNA testing. The HCV RNA level in blood helps predict the likelihood of a response to treatment, and the change in HCV RNA level can also be used to monitor the therapeutic response.
The same quantitative test should be used throughout therapy to avoid confusion, and results should be reported in international units to standardize data. The Versant HCV RNA Assay, version 3.0, is based on bDNA technology and has a dynamic range of 615-7,700,000 IU/mL. Another FDA-approved HCV quantitative test is the Aptima HCV Quant Dx Assay its limit of detection is 3.9 IU/mL in plasma and 3.4 IU/mL in serum.
The following are the best laboratory evidence of acute HCV infection:
- A positive HCV RNA test in the setting of a negative HCV antibody test
- A positive HCV antibody test after a prior negative HCV antibody test
It should be noted that impaired antibody production in immunosuppressed individuals may result in misleading information.
References
World Health Organization. Hepatitis C: fact sheet. Available at . Updated: October 2017 Accessed: January 23, 2018.
Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000 Mar 11. 355:887-91. .
Optimization Of The Qpcr Assay
The qPCR assay was optimized following the MIQE guidelines.2020 Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009 55:611-622. Primer and probe matrix experiments were conducted by selecting, for each gene, the primer concentration that resulted in the lowest cycle threshold and the highest ÎRn using a fixed amount of target template. Reactions with different concentrations of primers and probes were performed in total volumes of 25 µL and 12.5 µL, of TaqMan Universal Master Mix buffer sense primer and antisense primer concentrations of 50 nM, 300 nM, and 900 nM each probes at concentrations of 80 nM, 125 nM, 150 nM, and 250 nM and 1 µL of DNA or cDNA. The reaction was performed with a 7500 Real-Time PCR System using universal conditions: 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. For these reactions, an OptiQuant HBV/HCV Quantification Panel was used in the following concentrations expressed in international units/mL : HBV 7 or HCV 7 and HBV 2 or HCV 2 .
Read Also: Hepatitis C Ab W Reflex Hcv Rna Quant Rt Pcr
What The Quantitative Results Mean
The quantitative test results indicate the exact amount of HCV in your blood. This number helps your doctor confirm whether you have a high or low viral load.
Measuring your viral load before treatment allows your doctor to monitor your viral load during and after treatment.
The viral load measurement doesnt indicate how severe your HCV infection or cirrhosis is. Your doctor will need to take a biopsy, or tissue sample, from your liver to learn more about how your liver has been affected by an HCV infection.
The viral load results from the quantitative PCR test can range from 15 to 100,000,000 IU/L.
If your results are:
- Fewer than 15 IU/mL: The virus is detected, but the amount cant be measured exactly. You may need to return later for another test to see if the measurement changes.
- Fewer than 800,000 IU/mL: A low viral load is detected.
- More than 800,000 IU/mL: A high viral load is detected.
- More than 100,000,000 IU/mL: The virus is detected and active infection is taking place.
- Inconclusive: HCV RNA cant be measured, and a new sample needs to be taken.
The Quantitative Hcv Rna Test Is Checked Before A Patient Starts Treatment
For each patient, the result can be described as either a “high” viral load, which is usually > 800,000 IU/L, or a “low” viral load, which is usually < 800,000 IU/L. It’s not uncommon to have a viral load in the millions. Today’s hepatitis C treatments are very effective with both high and low viral loads. An undetectable HCV viral load 10-12 weeks after hepatitis C is completed is associated with a cure.
Read Also: Is There A Cure For Hepatitis A
Hepatitis C Virus Quantitative Real
The Quantitative, real-time PCR test measures the IU of the HCV RNA per millimeter of plasma or serum. This test is typically only done for known HCV positive individuals. If you are unsure or if you just want to screen for this virus, you may want to order the Hepatitis C Virus Antibody test.
Test results may take 3-5 business days.
Who International Standard For Hcv And Synthetic Hcv Positive Control
The 5th WHO International Standard for HCV was purchased from National Institute for Biological Standards and Control and reconstituted in 1.1mL of nuclease-free water to 100,000 IU/mL . The HCV Positive Control prepared in-house is a non-infectious recombinant MS2 encapsidated construct containing the same 92-base target sequence derived from genotype 1a HCV and is amplifiable by the same set of HCV specific primers for the assay. The concentration of the HCV Positive Control was calibrated with reference to the 5th WHO International Standard for HCV.
Recommended Reading: How Do You Get Hepatitis B And C
Sensitivity And Linearity Of The Real
The analytical sensitivity of our method was determined by serial dilutions of pFKI389-NS3-3 HCV subgenomic replicon plasmid containing from 0 to 20 × 106 copies tested 5 times each standard dilution was tested in triplicate. The detection limit was determined at 100 HCV copies per reaction with 100% detection ability . The correlation coefficient was 0.99 .
Sera For Hcv Rna Analysis
A total of 177 serum samples were obtained from the 59 patients. Each of the specimens was divided into four aliquots and frozen to 80°C within 2 h of collection . Each aliquot was used for HCV RNA quantitation by the following assays: Cobas TaqMan HCV , with a detection limit of 15 IU/ml , and the Abbott RealTime HCV quantitative assay , with a detection limit of 10 IU/ml.
The HCV RNA viral load was measured at the following three time points: baseline and 4 and 12 weeks after the beginning of therapy. SVR was determined 6 months after the end of therapy with the Amplicor qualitative assay . Values for HCV RNA are reported in IU/ml. All assays were done at Alphabio Laboratory.
Don’t Miss: How Do You Catch Hepatitis C
Hepatitis C Viral Load / Hcv Rna Quantitative Testing
Hepatitis C
The viral load of hepatitis C refers to the amount of virus present in the bloodstream. The quantitative HCV RNA tests measure the amount of hepatitis C virus in the blood. The result will be an exact number, such as “1,215,422 IU/L.” Many people refer to the quantitative measurement as the hepatitis C “viral load.”
Viral load tests are used to confirm active hepatitis C infection and are used during treatment to help determine response. If you have lower levels of virus in your blood when you start treatment, you may have a better chance of getting rid of the virus.
Preparation Of The Standard Curve
To perform an absolute quantitative qPCR, a plasmid construct harboring genotype 1b HCV subgenomic replicon was used to prepare the standard curve. We first calculated the mass of a single plasmid molecule based on the pFKI389-NS3-3 plasmid size, the mass of plasmid containing the copy number of interest that was 20,000,000 to 10 copies, and the concentration of plasmid needed to achieve the copy number of interest. This was followed by the preparation of 10-fold serial dilutions of plasmid template to create the standard curve. Finally, with the threshold cycle values, a linear regression model was calculated.
Read Also: Royal Canin Hepatic Dry Dog Food