Rflp Patterns And Hcv Genotyping
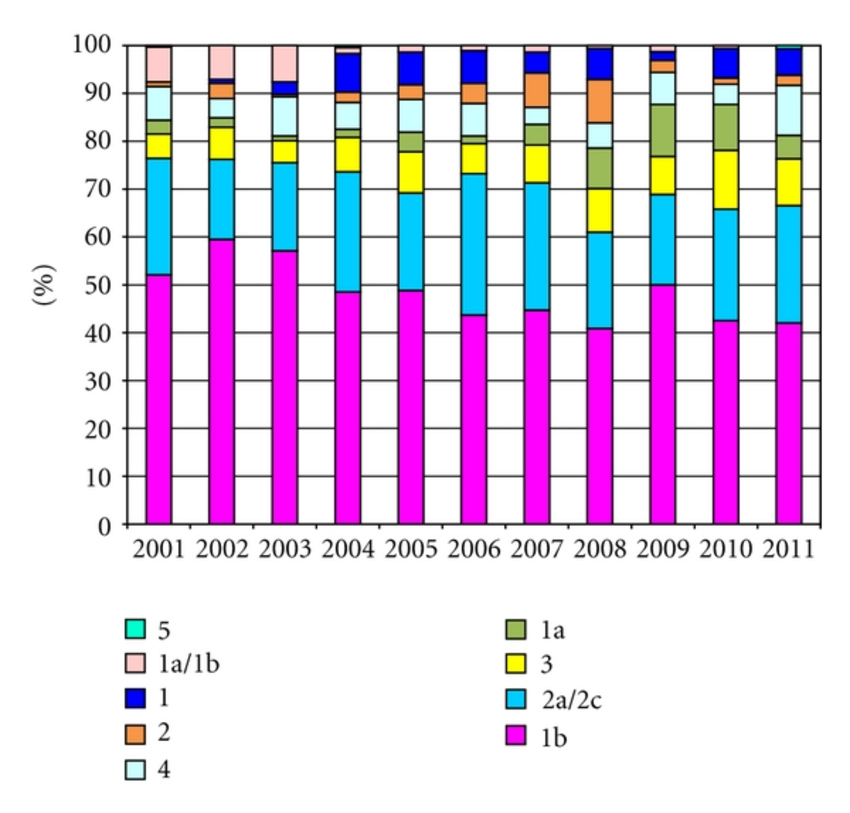
A total of 1,080 NCBI sequences were evaluated for genotype-specific RFLP patterns. The differentiation of the three main genotypes present in Brazil could theoretically be accomplished using a double digest with restriction enzymes BstN I andHinf I. The banding patterns of Genotype 3 samples consist of a 165bp fragment, while almost all genotype 1 samples contain a 117bp fragment and the majority of the genotype 2 samples have a 221bp fragment . To further differentiate HCV genotypes 4 and 5 and to differentiate a significant proportion of the genotype 2 samples that have a 117bp-fragment, the restriction enzyme Hae III was selected because it was informative for all of these discriminations. shows the predicted patterns using BstN I and Hinf I double digestion and Hae III single digestion for the HCV Brazilian sequences. Based on these results, the following workflow for genotyping HCV was defined: BstN I + Hinf I double digestion and Hae III single digestion in a first round for all samples. Samples with indeterminate patterns in the first round would be secondarily digested with Rsa I, BfuC I andBstU I.
TABLE 2
Question 6 Where Can I Find More Guidance For Testing Managing And Treating Hcv
The AASLD and IDSA recommendations for testing, managing, and treating HCV may be found at .
This FAQ is provided for informational purposes only and is not intended as medical advice. A clinicians test selection and interpretation, diagnosis, and patient management decisions should be based on his/her education, clinical expertise, and assessment of the patient.Document FAQS.21 Version: 2
Hepatitis C Virus Genotype 1 Subtype Identification In New Hcv Drug Development And Future Clinical Practice
-
Affiliations French National Reference Center for Viral Hepatitis B, C and delta, Department of Virology, Hôpital Henri Mondor, Université Paris 12, Créteil, France, INSERM U955, Créteil, France
-
Affiliations French National Reference Center for Viral Hepatitis B, C and delta, Department of Virology, Hôpital Henri Mondor, Université Paris 12, Créteil, France, INSERM U955, Créteil, France
-
Affiliation INSERM U955, Créteil, France
Recommended Reading: Is Viral Hepatitis C Contagious
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Hcv Detection And Quantification Validation Tests
First, an HCV-positive standard sample at a concentration of 10,000,000 IU/mL was diluted ten-fold to 1IU/mL, and the complete procedure was performed on all dilutions. Real-time PCR results obtained from the dilutions were linear and reproducible. High correlations were observed between samples with different viral loads, and the corresponding cycle threshold had a wide dynamic range of analysis across the entire spectrum of clinically relevant viral loads . Additionally, reproducibility was tested using four different HCV RNA genotype 1 samples in 20 runs on different days. The total coefficients of variation of the cycle threshold were 8.77% for sample A , 3.72% for sample B , 3.96% for sample C and 3.67% .
The limit of detection was estimated using the Probit test by evaluating nine replicates of seven diluted HCV RNA samples ranging from 51 to 3,300IU/mL. The assay sensitivities were 1,500IU/mL for 95% positive repetitions and 500IU/mL for 50% positive repetitions. Specificity was tested using hepatitis C-negative blood donors, and no false-positive results were observed in a total of 50 anti-HCV-negative samples.
FIGURE 1 –The relationship between HCV RNA viral load levels given by ControlLab and obtained by nested real-time RT-PCR. HCV RNA:RT-PCR:
Also Check: What Does Non Reactive Hepatitis B Mean
Hepatitis C Viral Load / Hcv Rna Quantitative Testing
Hepatitis C
The viral load of hepatitis C refers to the amount of virus present in the bloodstream. The quantitative HCV RNA tests measure the amount of hepatitis C virus in the blood. The result will be an exact number, such as “1,215,422 IU/L.” Many people refer to the quantitative measurement as the hepatitis C “viral load.”
Viral load tests are used to confirm active hepatitis C infection and are used during treatment to help determine response. If you have lower levels of virus in your blood when you start treatment, you may have a better chance of getting rid of the virus.
Serology And Molecular Analysis
All the plasma samples were analyzed for the presence of anti-HCV antibodies by means of the Vitros enhanced chemiluminescent immunoassay test , used according to the manufacturer’s instructions. This test employs chemiluminescent technology and results are reported as signal-to-cutoff . Results 1.00 were considered reactive for HCV antibodies. Specificity and sensitivity of the test were 99.97 and 100%, respectively.
As previously described and following CDC recommendations , anti-HCV-positive samples with S/Co ratios of 8.0 can be reported as positive without further supplemental testing. We therefore decided to include in this study only repeatedly anti-HCV-reactive samples with S/Co ratios of 8.0. These samples were subsequently tested for the presence of HCV RNA and its quantification was performed via COBAS AmpliPrep/TaqMan HCV 48 , which exploits a polymerase chain reaction in real time . Linear range of quantification of the test was 1.50 E+01 to 6.90 E+07 HCV RNA IU/ml, using the accuracy acceptance criterion of ±0.3 log10. Specificity of the test was 100% and its detection limit was 15 IU/ml.
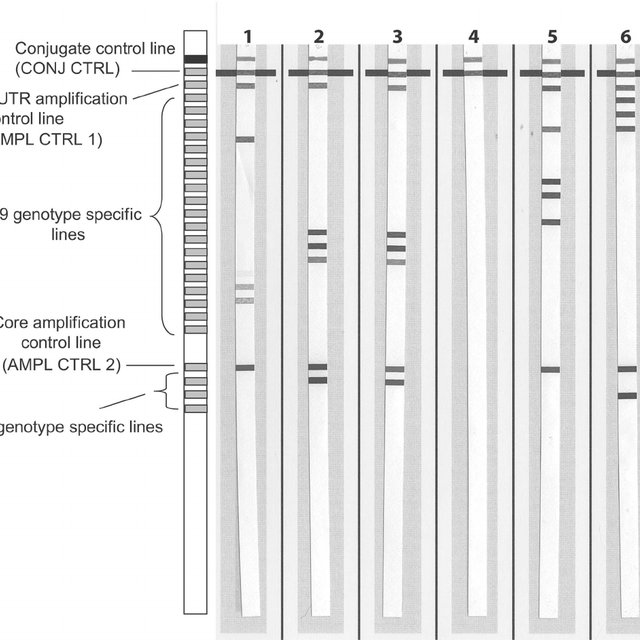
Fig. 1
Genotype-specific probes adsorbed onto nitrocellulose strips.
Don’t Miss: What Is Hepatitis B Virus
Question 5 How Does The Lipa Genotype Test Differ From The Hcv Ns3a Ns5a Or Ns5b Genotypic Tests For Drug Resistance
The LiPA genotype test is designed to detect all 6 major HCV genotypes. The NS3a, NS5a, and NS5b genotype tests, on the other hand, detect mutations associated with drug resistance they are limited to individuals infected with HCV genotype 1 or genotypes 1 and 3 in the case of NS5a and are not intended for determining HCV subtype. The Hepatitis C Viral RNA, Genotype, LiPA should be performed before ordering a drug resistance test.
Ial Sequencing Of Hcv Ns5b Region
Partial NSB5 sequencing was performed for 146 of the 304 specimens to verify the accuracy of the HCV genotypes determined by the triplex HCV genotype PCR reactions. Reverse transcription was performed essentially as previously described using 300 U of Moloney Murine Leukemia Virus RT and 7.5 ng/µl of random primer .
Primers NS5B2F and NS5B-2R , located between the positions 1683 and 1704 of the NS5B region, were used for partial sequencing of the NS5B region generating an expected product of 688 bp. Each PCR reaction contained buffer , 1.5 mM MgCl2, 200 nM of each dNTP, 1 µM of each primer, 2.5 IU of High Fidelity Taq DNA polymerase , and 5 µl of cDNA to be tested. The final volume was adjusted to 50 µl using ultrapure water. PCR was carried out using a GeneAmp PCR System 9700 . The following amplification conditions were used: 5 min at 94°C 35 cycles at 95°C for 30 s, 60.5°C for 1 min for primers NS5B2F/NS5B2R or 56°C for 45 s for primers F56_13/R56_13 , 72°C for 1 min 10 min at 72°C and a final hold at 10°C.
Sequencing reactions were performed in both directions using the Big Dye terminator technology and products were detected with an ABI 3130 Genetic Analyzer . Sequencing analysis was performed using SeqScape , and genotypes were assigned by matching using Blast .
The generated sequences were submitted to GenBank and can be retrieved under accession numbers FJ159697 to FJ159849.
You May Like: Can You Die With Hepatitis C
Development Of Hepatitis C Virus Genotyping By Real
-
Affiliations Laboratório Central do Estado , São José dos Pinhais, Paraná, Brazil, Department of Gastroenterology, School of Medicine, University of São Paulo , São Paulo, Brazil
-
Affiliation Centro de Genomas, São Paulo, São Paulo, Brazil
-
Affiliations Laboratório Central do Estado , São José dos Pinhais, Paraná, Brazil, Departamento de Biologia Celular, Universidade Federal do Paraná, Curitiba, Brazil
-
Affiliation Instituto Carlos Chagas Fundação Oswaldo Cruz , Curitiba, Paraná, Brazil
-
Affiliation Instituto Carlos Chagas Fundação Oswaldo Cruz , Curitiba, Paraná, Brazil
-
Affiliation Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, Indiana, United States of America
-
Affiliations Department of Veterinary Medicine, Federal University of Paraná, Curitiba, Brazil, Department of Pathobiology, University of Illinois, Urbana, Illinois, United States of America
-
Affiliation Department of Gastroenterology, School of Medicine, University of São Paulo , São Paulo, Brazil
-
Affiliation Department of Gastroenterology, School of Medicine, University of São Paulo , São Paulo, Brazil
Other Things To Know:
- The viral load measurement does not tell us anything about the severity of a patient’s liver disease or the degree of fibrosis . For that information, the patient would need additional testing.
- It is not necessary to check the viral load repeatedly during treatment.
- If a quantitative HCV RNA result is reported as “< 15 IU/L,” this means that the quantitative test cannot measure the hepatitis C virus. It may mean that there is no detectable HCV RNA at all, but it may mean that the level of virus is just too low for the test to pick it up.
Don’t Miss: What Is The Definition Of Hepatitis B
Cautions Discusses Conditions That May Cause Diagnostic Confusion Including Improper Specimen Collection And Handling Inappropriate Test Selection And Interfering Substances
An “Undetected” or “Indeterminate” hepatitis C virus genotype result does not rule-out active HCV infection. Test results should be correlated with routine serologic and molecular-based testing, as well as clinical presentation. Specimens with indeterminate results will be automatically evaluated with the subsequent test HCVGR / Hepatitis C Virus Genotype Resolution, Serum.
Known cross-reactivity between the assay probes and various HCV genotypes limits the ability of this assay to identify multiple HCV genotypes present in a given specimen. Such cross-reactivity or the actual presence of multiple HCV genotypes in the same specimen may result in an “Indeterminate” or multiple/mixed genotype result.
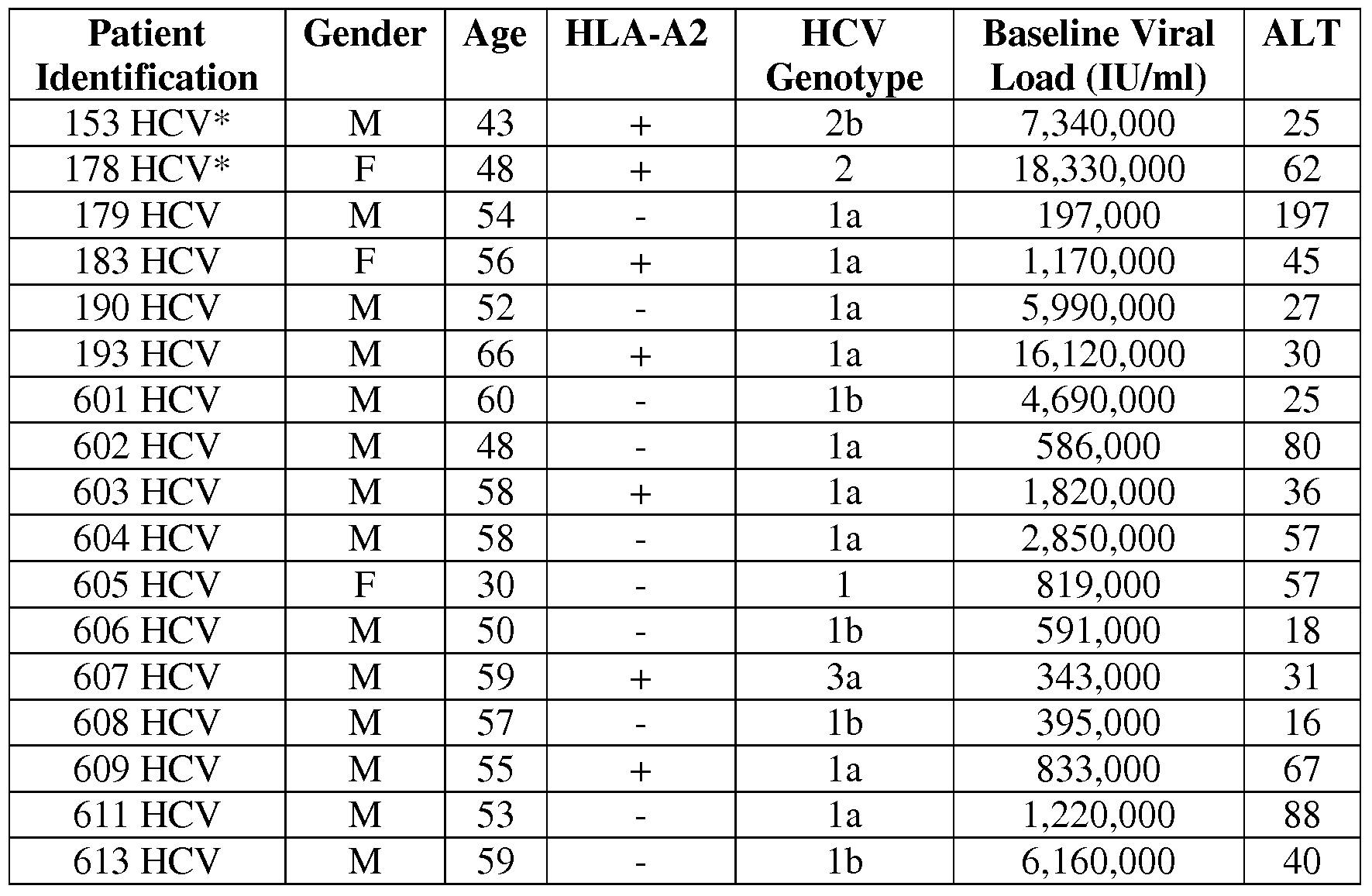
Study Population And Sample Collection
From a total of 947 anti-HCV-positive patients, consecutively recruited among patients referred to ambulatories of virology of the National Cancer Institute Fondazione G. Pascale’, IRCCS Italia and of the Second University of Naples SUN, Section of Infectious Diseases, Naples, Italy, between January 2012 and April 2013, we included 475 anti-HCV/HCV RNA-positive patients , all coming from different cities/towns of the Campania region. All the remaining 472 patients were HCV-RNA-negative and therefore were excluded from the study.
The ethics committee reviewed the study which was conducted following the principles of the ICH GCP and Declaration of Helsinki. All subjects provided written informed consent. All data was de-identified during data collection.
All patients enrolled were asymptomatic, negative for anti-HIV, HBsAg and anti-HDV and had no clinical or serological signs of other chronic liver disease . No patient admitted alcohol abuse that was defined as the consumption of alcohol exceeding 30 g/day for females and 40 g/day for males in the last 6 months.
You May Like: How To Find Out If You Have Hepatitis
Hepatitis C Virus Genotype Serum
Determining hepatitis C virus genotype to guide antiviral therapy in patients with chronic hepatitis C
Differentiating between HCV subtypes 1a and 1b
This assay should notbe used as a screening test for HCV infection. It should be performed only on specimens obtained from patients confirmed to have HCV RNA levels in serum of 500 IU/mL or higher.
Laboratory Quality Assurance And Monitoring
Serum samples were processed, stored, and shipped to the Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA for analysis.
Detailed instructions on specimen collection and processing are discussed in the NHANES Laboratory Procedures Manual . Vials are stored under appropriate frozen conditions until they are shipped to Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention for testing.
The NHANES quality assurance and quality control protocols meet the 1988 Clinical Laboratory Improvement Act mandates. Detailed QA/QC instructions are discussed in the NHANES LPM.
Mobile Examination Centers
Laboratory team performance is monitored using several techniques. NCHS and contract consultants use a structured competency assessment evaluation during visits to evaluate both the quality of the laboratory work and the quality-control procedures. Each laboratory staff member is observed for equipment operation, specimen collection and preparation testing procedures and constructive feedback are given to each staff member. Formal retraining sessions are conducted annually to ensure that required skill levels were maintained.
Analytical Laboratories
You May Like: Hepatitis C Symptoms Mayo Clinic
Low Levels Of Hepatitis C Virus Rna In Serum Plasma And Peripheral Blood Mononuclear Cells Of Injecting Drug Users During Long Antibody
Marcel Beld, Maarten Penning, Marieke van Putten, Anneke van den Hoek, Marjolein Damen, Michel R. Klein, Jaap Goudsmit Low Levels of Hepatitis C Virus RNA in Serum, Plasma, and Peripheral Blood Mononuclear Cells of Injecting Drug Users During Long Antibody-Undetectable Periods Before Seroconversion. Blood 1999 94 : 11831191. doi:
Sequences And Comparative Analysis
A total of 1,080 HCV 5UTR nucleotide sequences were retrieved using Entrez from the National Center for Biotechnology Information . These sequences were obtained from previous HCV genotype prevalence studies with 1,080 different HCV infected patients from different states of the 5 geographic regions from Brazil2727. Campiotto S, Pinho JRR, Carrilho FJ, Silva LC, Souto FJD, Spinelli V, et al. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res 2005 38:41-48.. Additional 26 reference sequences from different genotypes were retrieved for comparative analysis66. Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005 42:962-973..
Sequences were edited and aligned with EditSeq and MegAlign programs from the DNAstar package . Primers and one probe were selected directly from the aligned sequences. The presence of the restriction sites for Hae III, Hinf I, BstN I,Rsa I, BfuC I and BstU I was determined using Mapdraw program within the DNAstar package .
Don’t Miss: Can You Get Hepatitis C From Drinking After Someone
What Tests Are Needed For Knowing My Genotype
When screening for HCV, a patient takes several tests to make a diagnosis .
Once a patient has been diagnosed with HCV, the doctor will run viral load level and genotype tests before starting treatment. Knowing a patients genotype determines the best treatment regimen.
Genotype tests use blood taken from fingersticks or simple blood draws. A patient might need to return to the doctors office to confirm whether the infection is chronic or to confirm whether they have been cured of the virus.
Genotypes 1a and 1b may require a patient to take additional blood tests to determine whether the virus has any resistance .
HCV treatment is now simpler, safer, and more effective, and diagnostics, including HCV genotyping, need to become simpler and less expensive.
The medications are available depending on the payer or what is available in a country or region.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Read Also: How Much Does A Hepatitis A Shot Cost
In Vivo Tropism Of Hepatitis C Virus Genomic Sequences In Hematopoietic Cells: Influence Of Viral Load Viral Genotype And Cell Phenotype
Herve Lerat, Sylvie Rumin, Francois Habersetzer, Francoise Berby, Mary-Anne Trabaud, Christian Trepo, Genevieve Inchauspe In Vivo Tropism of Hepatitis C Virus Genomic Sequences in Hematopoietic Cells: Influence of Viral Load, Viral Genotype, and Cell Phenotype. Blood 1998 91 : 38413849. doi:
Unitary Test Cost Estimation
Estimation of unitary test costs was based on the currently available commercial reagent prices in Brazil at the time the study was conducted. Equipment maintenance, human resources and other indirect costs were not taken into account for comparison and calculation.
To estimate the unitary cost of the real-time PCR test, costs of NucliSens EasyMAG extraction kit , Superscript III Platinum One-Step qRT-PCR kit for 500 reactions , TaqMan probes , primer sets , RNase Out , and optical tubes and caps were taken for comparison. Assessment of the final costs related to LiPA v.1 included the Amplicor® Hepatitis C VírusHVCTest, version 2.0 and the VERSANT HCV Genotype Assay-LiPA v.1 .
Read Also: Is There A Shot For Hepatitis C
What To Expect During Testing
A healthcare provider will take a blood sample for analysis.
Before the test, let them know if youre uncomfortable with certain needles or if youve ever passed out at the sight of blood. They can give you a snack to reduce your risk of fainting.
The needle may sting a little as it enters your skin, and you may have a bruise on the site of the draw for a few days.
Results are usually available within a few days or a few weeks at most.
The HCV RNA PCR test is conducted through a process called polymerase chain reaction . There are two approaches to this process: qualitative and quantitative.