Hepatitis C Signal To Cut Off
- Jie PanNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
- Xin LiNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
- Guowei HeThe Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, China
- Shuai YuanNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
- Pingfeng FengNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
- Xiuming ZhangZhongshan Hospital Affiliated to Sun Yat-Sen University, Zhongshan, Guangdong, China
- Yurong QiuNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
Hepatitis C Antibody With Reflex To Pcr
- Hepatitis C Ab w/RFLX PCR
- Lab Code
- Hepatitis C Antibody w/Reflex PCR
- Description
-
The Qualitative detection of Hepatitis C virus IgG and IgM antibodies in human sera by the FDA approved Abbott ARCHITECT Anti-HCV test two-step chemiluminescent immunoassay.
In the first step, sample, assay diluent, and recombinant HCV antigen coated paramagnetic microparticles are combined. Anti-HCV present in the sample binds to the rHCV coated microparticles. In the second step, anti-human IgG/IgM acridinium-labeled conjugate is added, which binds to IgG and IgM anti-HCV. Then pre-trigger and trigger solutions are added to the reaction mixture. The resulting chemiluminescent reaction is measured as relative light units .
The presence or absence of IgG/IgM anti-HCV in the sample is determined by comparing the chemiluminescent signal in the reaction to the cutoff signal determined from an ARCHITECT Anti-HCV calibration. Specimens with signal to cutoff values 1.00 are considered reactive for IgG/IgM anti-HCV. Specimens with S/CO values < 0.79 are considered nonreactive and specimens with S/CO values between 0.80 and 0.99 are Indeterminate.
Reactive anti-HCV will reflex to Hepatitis C RNA, Quantitative for confirmation with an additional charge.
For anti-HCV testing without PCR reflex for REACTIVE results, see Hepatitis C Antibody without PCR reflex on reactive samples .
- Synonyms
Other Hepatitis C Tests
After an individual has received a reactive or positive result from a hepatitis C antibody test, they will need to have two follow-up tests.
The first test checks to see whether a person still has the virus the other measures the amount of the virus in the blood.
The first test is the hep C RNA qualitative test, also known as the PCR test. A positive result means that a person has the hepatitis C virus. A negative result means that the body has cleared the virus without treatment.
The second test is the hep C RNA quantitative test. The result of this test is given as a number rather than a positive or negative. This is because the test compares the amount of the virus in the body before, during, and after treatment.
The number given as a result of this test is known as the viral load. The lower amount of the hepatitis C virus in the blood, the better the chances that a person can eliminate the virus from their body.
After hepatitis C virus is diagnosed, other tests may be needed:
Certain behaviors, experiences, and medical procedures increase the risk of getting the hepatitis C virus, which is transmitted by contact with blood.
The following are risk factors for contracting the virus:
The Centers for Disease Control and Prevention advise all baby boomers get tested for hepatitis C. Baby boomers are people born between 1945 and 1965. They are five times more likely to have the virus than other adults.
Recommended Reading: What Does Hepatitis B Do To You
Recommended Reading: Home Remedies For Hepatitis C In Urdu
What Do The Results Mean
There are two results from a hepatitis C antibody test.
- A non-reactive or negative test result means that the person does not have the virus. The exception is if someone has come into contact with the virus recently, such as through contaminated blood. If this is the case, they will need to have another test.
- A reactive or positive test result means that the person has had the virus at some point but does not mean that they still have it. Further tests will be needed to check whether the virus is still active in the body and if treatment will be required.
Once diagnosed with hepatitis C, a person will need to undergo a series of different tests to see how the virus has affected their body.
These tests will check for any liver damage, identify how well the liver is working, and help a healthcare professional to decide on treatment.
Hepatitis C is treated with medication known as an antiviral. It gets this name because it aims to clear the virus out of the body.
Another aim of the medication is to slow down damage to the liver. It may also reduce the chance of a person getting liver cancer or developing serious liver scarring, known as cirrhosis.
A person with hepatitis C will require regular testing during treatment to see how well the medication is working. Keeping healthy, getting enough sleep, and avoiding drugs and alcohol can help treatment to work.
Donât Miss: Is There Any Treatment For Hepatitis B
The Usefulness Of Anti
Farzaneh Tavassoli1
1Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, IR Iran
How to Cite: Ranjbar Kermani F, Sharifi Z, Ferdowsian F, Paz Z, Tavassoli F. The Usefulness of Anti-HCV Signal to Cut-off Ratio in Predicting Viremia in Anti-HCV in Patients With Hepatitis C Virus Infection. Jundishapur J Microbiol. 2015 8:e17841. doi: 10.5812/jjm.82015.17841.
ARTICLE INFORMATION
Read Also: Hepatitis C And Kidney Failure
Read Also: What Kind Of Disease Is Hepatitis C
Detection Of Hepatitis C Viral Load
The Real-Time HCV assay was performed to quantify the HCV viral load in all the specimens. The assay had a linear range of 12 IU/mL to 100 million IU/mL . The Limit of Detection of this assay is 12 IU/mL , equivalent to the lower limit of quantitation . The sensitivity for the assay was 12 IU/mL for the 0.5 mL specimen volume and the specificity was 99.5%. HCV viral load1.08 log IU/mL was considered as positive.
Individual Donar Nucleic Acid Testing
For ID-NAT, Procleix Ultrio kit was used based on TMA. The assay contains reagents which are used for simultaneous detection of all three viruses initially. Initial NAT assay was done on the pilot tube sample and if found reactive then the sample from the bag was repeated twice. The repeat sample testing if found reactive, was further tested by discriminatory testing for HBV, HCV, and HIV, respectively. A positive discriminatory test confirmed the presence of the respective virus. The clinical sensitivity for the Procleix Ultrio Assay has been demonstrated for specimens with HIV-1 or HCV viral RNA concentrations equal to or > 100 copies/ml or HBV viral DNA concentrations equal to or > 15 IU/ml.
Also Check: Tested Positive For Hepatitis B
Hepatitis C Virus Antibody
Hepatitis C virus infection is the most common chronic blood-borne infection in the United States. It is estimated that 40% of chronic liver disease is HCV related and HCV-associated end-stage liver disease is the most frequent indication for liver transplantation among adults.
Acute hepatitis C is generally a benign disease. In transfusion transmitted infections, where the acute onset is best documented, 70% of cases are anicteric and asymptomatic 30% have a bilirubin greater than 2.5 mg/dL and the mean peak ALT is 700 U/L. However, patients with community acquired acute HCV, usually present with overt clinical illness 70% are icteric and 75% have ALT levels that exceed 15 times the upper limit of normal. Approximately 50% of infected individuals evolve into chronic liver disease. Chronic hepatitis C infection is the primary indication for liver transplantation.
In 1998, CDC recommended HCV testing for individuals at high risk for HCV transmission, including those who had injected drugs, been hemodialysed, transfused or transplanted before July 1992, or received clotting factor concentrates produced before 1987. Screening also was recommended for persons with occupational sharps exposures, children born to HCV-infected mothers and individuals with persistently elevated ALT levels and individuals infected with HIV. :
1. Persons who ever injected illegal drugs
Limitations Of Our Study
The kits used for ELISA were only from a single manufacturer hence results of predictive value may change when other kits are used. Even confirmation of ELISA anti-HCV status with other ELISA platforms should have been performed instead we confirmed the infection status with NAT. An ideal study design would have been to use an unscreened population for all available kits and then perform supplemental tests and S/CO analysis on those repeat reactive.
Recommended Reading: How Does Hepatitis C Start
Recombinant Immunoblot Assay For Anti
Specimens with reactive anti-HCV results were further tested with RIBAs to confirm anti-HCV positivity using a recombinant immunoblot kit for antibodies against the hepatitis C virus . The nitrocellulose strips contained seven bands for the core, NS3, NS4-1, NS4-2, and NS5 antigens as well as control A and control B. The result was defined as negative,±, 1+, or 2+by comparing the colour of the antigen band with control A. Anti-HCV positivity was defined as the presence of at least two antigens with greater than or equal to 1+ reactivity. An indeterminate result was defined by only one band scoring1+. Anti-HCV negativity was defined by the absence of antigens scoring1+. The tests were performed according to the manufacturers instructions by technicians with more than 10 years of experience.
Test Procedure And Results Of Patient Samples
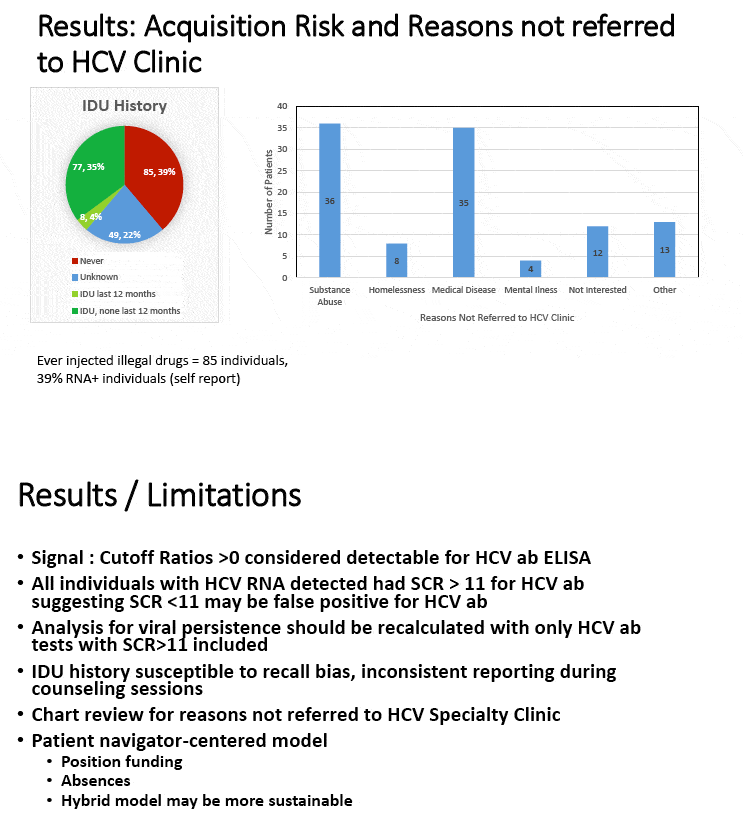
Among 719 PWIDs samples, 416 yielded reactive results and 303 yielded negative results by ELISA assays. Moreover, the results of supplementary test by RIBA assays showed that of the 416 ELISA screening test reactivity samples, 393 were positive for HCV antibodies, 7 were indeterminate, and 16 were negative. In addition, HCV RNA results revealed that of the 400 HCV antibody positive or indeterminate samples, 315 were positive for HCV RNA and 85 were negative for HCV RNA. Furthermore, 6 out of 319 HCV antibody negatives were positive for HCV RNA, implying that 6 of the 319 individuals in this study had HCV acute early infection or immunodeficiency. The HCV test assays and test results were summarized in Fig. 1.
Fig. 1
Schematic diagram of the HCV detection methods and sample number.719 PWIDs samples were collected for screening test by ELISA assays, and then supplementary test by RIBA assay and NAT assay to determine the status of HCV infection
Read Also: Hepatitis C Transmission Routes Cdc
Ecl Immunoassay For Anti
An ECL immunoassay was applied for anti-HCV testing using the Elecsys anti-HCV II assay on the Cobas 601 analyser . The kit was a third-generation test using peptides and recombinant antigens representing the core, NS3, and NS4 to capture the corresponding antibodies. The results were expressed as signal-to-cutoff ratios: S/CO< 1.0 indicated anti-HCV nonreactivity, and S/CO1.0 indicated anti-HCV reactivity. All S/CO1.0 sera were retested in duplicate according to the manufacturerâs instructions. If either of the two results remained S/CO1.0, then the subject was considered anti-HCV reactive. The anti-HCV reactive sera were retested using an Architect anti-HCV reagent kit.
Screening For Hepatitis C Virus
All donations were tested for anti-HCV and HCV RNA as per algorithm 1. All donations were tested in parallel and if results of NAT and ELISA do not match the samples for further evaluation were stored from the plasma bag. Any IR result was repeated again on the sample from the bag and pilot tube before labeling it as repeat reactive . Any sample which was not RR on ELISA by pilot tube and bag, was considered as contamination.
All ELISA and NAT nonreactive samples were considered as concordant nonreactive for HCV whereas ELISA and NAT reactive donor sample was considered as concordant positives. Bag and samples were quarantine and discarded.
Any sample which was HCV NAT reactive but ELISA nonreactive was considered as NAT yield for HCV where as a ELISA reactive and NAT nonreactive sample was referred as sero-yield. All sero-yield samples were further tested with a rapid assay, fourth generation, quantitative immunoassay , for anti-HCV detection.
Read Also: New Medicine For Hepatitis B
Comparing The Four Anti
The four assays showed great performance in diagnosing viremia specimens and all 264 HCV RNA specimens displayed positive anti-HCV results. In this HCV RNA group, the two CIA assays generated overall higher anti-HCV S/Co values than the two EIA assays. The S/Co values in this group were all greater than 4 using Elecsys-ECLIA. Except one sample between 1 and 2.5, the S/Co values were all greater than 4 using Ortho-ELISA. Except two samples between 2.5 and 4, the S/Co values were all greater than 4 using Architect-CMIA. The S/Co values from Murex-ELISA were higher than 4 in 213 specimens , between 2.5 and 4 in 29 specimens , and between 1 and 2.5 in 22 specimens .
Table 1 HCV infection status in relation to four S/Co ranges in four assays