Reasons To Delay Treatment
Pregnancy
Hepatitis C treatment is generally not recommended during pregnancy. There is not a lot of information on the effects of DAAs during pregnancy. Research is being done on taking DAAs during pregnancy so this may change in the future.
Treatment that includes ribavirin can cause severe birth defects and must not be taken during pregnancy. When a couple wants to have a baby, both partners should avoid using ribavirin for at least six months before trying to get pregnant.
A healthcare provider can help determine a treatment plan and timeline for a person who has hepatitis C and wants to have a baby.
Children and adolescents
Hepatitis C treatment for children over the age of 12 is available in Canada. It is recommended that children who require treatment for hepatitis C be connected to a specialist with experience treating the pediatric population.
Resources for service providers
Monitoring Patients Who Are Starting Hcv Treatment Are On Treatment Or Have Completed Therapy
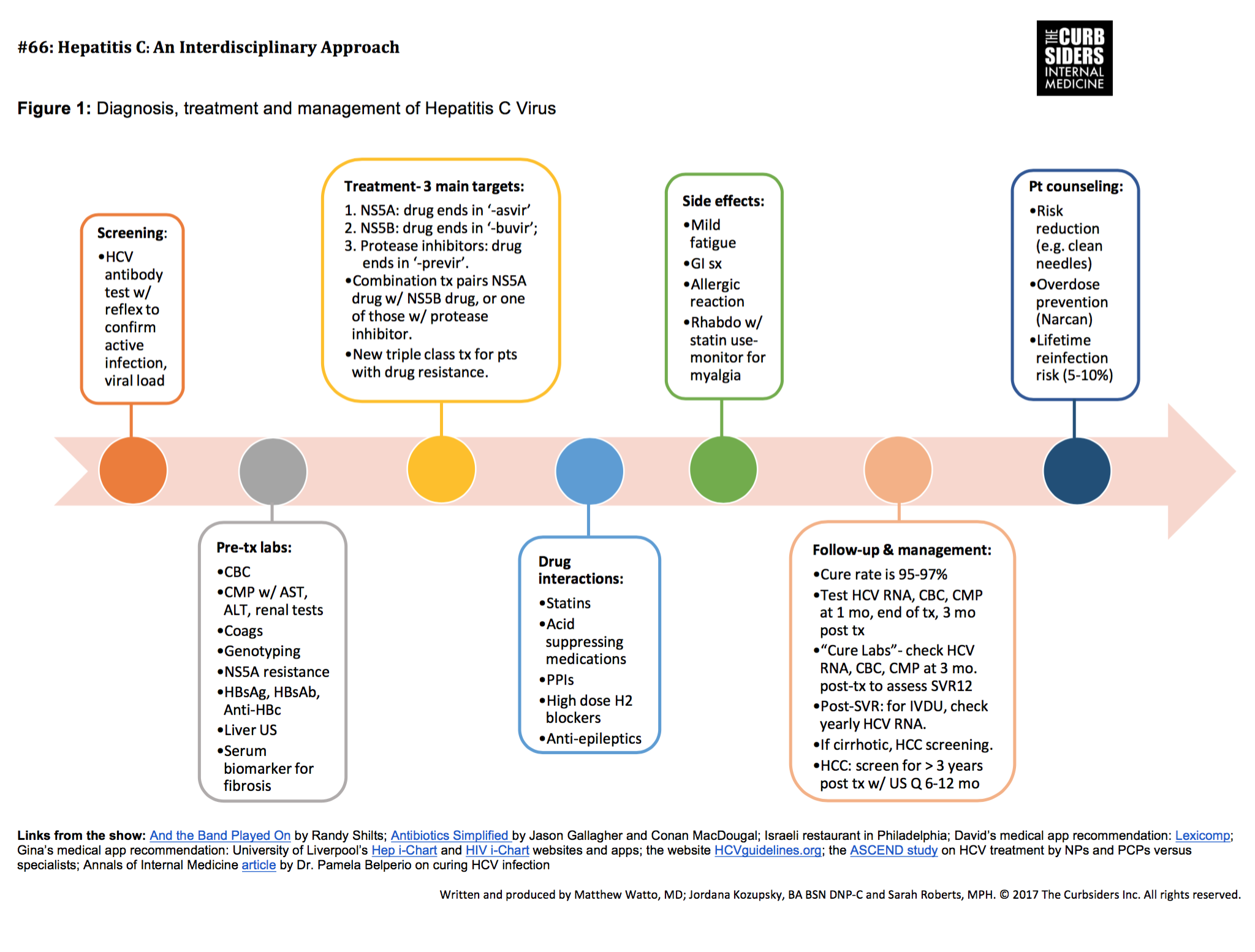
This section provides guidance on monitoring patients with chronic hepatitis C virus infection who are starting direct-acting antiviral treatment, are on treatment, or have completed therapy. It is divided into 4 parts: pretreatment and on-treatment monitoring posttreatment follow-up for persons in whom treatment failed to clear the virus posttreatment follow-up for those who achieve a sustained virologic response and additional considerations if treatment includes ribavirin.
Clinical Pharmacology Of Newer Daa
The direct acting antiviral therapy was started when the first-wave, first-generation HCV NS3-4A protease inhibitors boceprevir and telaprevir were approved in combination with PegIFN-alpha and ribavirin for the treatment of chronic HCV genotype 1 infection in 2011.However, these drugs have been reported to have several drug-drug interactions, and are largely replaced with newer DAA including Simeprevir, Paritaprevir, Daclatasvir, Ledipasvir, Ombitasvir, Sofosbuvir and Dasabuvir. summarizes the pharmacokinetics of all approved DAA with special reference to the sites of drug-drug interaction. Clinical pharmacology of the newer DAA will be discussed in the next section.
Recommended Reading: What Is Chronic Viral Hepatitis C
Study Design And Participants
This observational cross-sectional study recruited individuals aged 18 years or older with a history of injecting drug use who had achieved the end of treatment response to any interferon-free DAA treatment between 2015 and 2020. The study was conducted in three centers. There were two centers in Flanders, one out of hospital drug addiction center and the digestive department of a hospital. The third center, a hepatologist private practice , was in Brussels.
The local researchers of the cooperating centers contacted the potential study participants and invited them to participate in the study between August 2019 and December 2020. A blood draw was done during the study visit for HCV RNA determination. Potential HCV risk factors were assessed through a face-to-face questionnaire on paper in a private and secure setting. The questionnaire was available in Dutch, French, and English and covered a total of 29 questions. Data from the questionnaire included birth gender, year of birth, source of income, level of education, housing in the past six months, history of imprisonment, tattoos or piercings placed in a potentially non-sterile environment, number of unsafe sexual partners, frequency of alcohol abuse , age of first drug use, kind of drugs injected and when , frequency of IDU after DAA treatment, having shared paraphernalia, OAT, and needle syringe program . If available in the medical record, the results of the FibroScan® were noted .
Treatment For Acute And Chronic Hepatitis C Infection
Some people are diagnosed with hepatitis C when the infection is in the acute phase . About one in four people will clear the hepatitis C virus from their body on their own within six months. When a person is diagnosed with hepatitis C in the acute phase, a healthcare provider might recommend waiting to see if their body clears the virus on its own.
Current treatment guidelines in Canada focus on treatment for chronic hepatitis C infection . The treatment guidelines recommend that treatment of acute hepatitis C infection be assessed on an individualized basis. In many cases, a person has to progress to chronic hepatitis C infection before they can receive public or private drug coverage for hepatitis C treatments.
Don’t Miss: How Do You Contact Hepatitis A
Current Antiviral Treatment Strategies
The introduction of DAA revolutionized the field of antiviral therapy for patients chronically infected with HCV. Antiviral therapy usually consists of at least two antiviral substances from different drug classes with different modes of action . Treatment decisions are based on genotype , presence of cirrhosis and response to prior treatments . Typical treatment regimens for patients with and without compensated cirrhosis are depicted in Tables 1 and 2. All different recommended regimens achieve SVR rates of more than 95% if administered correctly .
Table 1.
Treatment of patients with chronic hepatitis C without cirrhosis
Table 2.
Treatment of patients with chronic hepatitis C with compensated cirrhosis
Fig. 1.
The replication cycle of the hepatitis C virus and modes of action of direct-acting antivirals are displayed .
The pangenotypic drug combinations sofosbuvir/velpatasvir and glecaprevir/pibrentasvir show high antiviral efficacy against all HCV genotypes. Treatment duration differs from 8 weeks for glecaprevir/pibrentasvir in noncirrhotic treatment-naive patients to 12 weeks for patients with liver cirrhosis or 16 weeks for GT 3 patients with liver cirrhosis and/or prior treatment failure. Sofosbuvir/velpatasvir has to be administered for 12 weeks independently of fibrosis level . Treatment with grazoprevir/elbasvir is possible in patients with GT 1 or 4 infection and has to be administered for 1216 weeks depending on GT, fibrosis stage and viral load .
Evaluations Of Efficacy And Safety
The primary efficacy endpoint was sustained virologic response . Adverse events and serious adverse events that occurred both during and after treatment were recorded by physicians or nurses in charge. AEs related to DAAs therapy was defined as any unintended and unfavorable sign , symptom, or disease temporally associated with the use of DAA drugs. DAA drugs and these adverse reactions followed a chronological order . All AEs were classified according to the Common Terminology Criteria for Adverse Events Version 5.0 developed by the US National Cancer Institute . Based on the tool, the severity of adverse event was classified according to unique clinical descriptions for each event.
Read Also: What Is Hepatic Function Panel
Challenges And Future Direction Of Daa
Though DAA has provided much needed, safe and effective therapeutic option for chronic HCV patient, some challenges need further effort. Such challenges include the presence of resistant variance, low efficacy in cirrhotic patients, presence of drug-drug interactions, and the cost. The future direction should go through multiple directions, for example, continuous monitoring and developing DAA, use of combined groups of DAA with different mechanisms of action to minimize resistance, searching for other antiviral groups with different mechanisms of action, and finding a solution for improving cirrhosis by developing antifibrotic drugs. A recent study showed promising results in the possible incorporation of a new cyclophilin inhibitor, STG-175 in DAA-regimen.
Study Population And Antiviral Regimens
In this single-centerTianjin Second Peoples Hospitalretrospective real-world cohort study, patients meeting the following inclusion criteria were enrolled: 18 years old a history of chronic HCV infection HCV GT 1, 2, 3, 6, unknown, or mixed with or without cirrhosis treatment-naïve or treatment-experienced with interferon-based regimens negative results for antinuclear, anti-mitochondria, anti-smooth muscle autoantibodies with Tianjin local Medical Insurance treated with available brand DAAs covered by Tianjin local health insurance. Exclusion criteria were as listed: incomplete data, discontinued treatment, or loss during the 12-week post treatment follow-up. Ethical approval was obtained from the human medical ethics committee of Tianjin Second Peoples Hospital and carried out following the principles of the Helsinki Declaration. Written informed consent was provided by each recruited patient.
Don’t Miss: What Do You Get Hepatitis C From
Treatment Of Patients With Prior Daa Treatment Failure
After failure of DAA treatment, the development of RAS is very likely. RAS linked to the NS5A gene are more likely to persist for a longer time at a significant level than those connected to the NS3/4 gene. RAS relevant for the NS5B inhibitor sofosbuvir are usually rapidly suppressed after treatment cessation due to a significantly impaired viral fitness. Resistance testing and the adaptation of antiviral regimes according to RAS is one possible option for retreatment . Currently, the only approved drug combination for the retreatment of patients with prior DAA failure is the combination of sofosbuvir, velpatasvir and voxilaprevir . This combination is not only well tolerated, but also highly effective, and SVR rates > 95% can be achieved in pretreated patients independently from the initial treatment regimen . The recommended treatment duration for DAA-experienced patients is 12 weeks . Preliminary real-world data confirmed the high efficacy of the SOF/VEL/VOX in previous DAA failures. All of the first 110 patients who had completed SOF/VEL/VOX in the German Hepatitis C Registry achieved SVR . Excellent results have also been reported from French and US cohorts . Due to its effectiveness this combination should be reserved for the retreatment of patients with prior DAA failure and is usually not recommended for the initial treatment of therapy-naive patients .
Recovery Of Metabolic Damage
The association between HCV infection and dysregulation of metabolic processes has been observed since long ago. Furthermore, chronic HCV infection exerts a significant impact on the development of heart disease and stroke . Increasing epidemiological studies have long demonstrated that the prevalence of type 2 diabetes mellitus is much higher in subjects with chronic hepatitis C than in the general population, ranging between 13% and 67% according to liver fibrosis stage and time of infection .
The hypothesis that HCV has a direct and important role in the regulation of glucose metabolism is supported by laboratorial investigations. Kasai D et al. showed that HCV replication can down- regulate the glucose transporter 2 expressions, which located on the surface of the cell, thereby affecting cellular uptake of glucose . Deng L et al. found that HCV up-regulated hepatic glucose production via NS5A-mediated FoxO1-dependent pathway . Recently, more systematic mechanisms underlying disorders of glucose metabolism caused by HCV infection have been observed in many experimental and clinical studies. HCV may directly inhibit the insulin-signaling pathway, with downregulation of glucose transporter 2, promotion of IRS-1 degradation through protein kinase B /mammalian target of rapamycin activation, and suppression of phosphorylation of tyrosine on IRS-1. Moreover, HCV impairs phosphorylation of Akt, leading to a reduction in insulin stimulation .
Figure 1
Recommended Reading: Is Hepatitis Ca Sexually Transmitted Disease
Pharmacologic Basis Of Direct Acting Antiviral Drug Interaction
Drug-drug interaction is the modification of the action of one drug by another and may be pharmacodynamic or pharmacokinetic in nature. Pharmacodynamic interactions dont result due to a change in drug concentrations but lead to alteration of the response of body to another drug. Pharmacodynamic interactions may occur between drugs with opposite actions leading to antagonism or may occur between drugs with similar actions leading to potentiation, addition or synergism. Pharmacodynamic interactions are not easy to be quantified, and subsequently, are difficult to manage. For example, during the interferon-based therapy for chronic HCV, Peg-INF enhances the ribavirin-induced anemia to discontinue the therapy. On the other hand, pharmacokinetic interactions do result in a change in drug concentrations and occur at the level of drug absorption, distribution, metabolism and elimination. Pharmacokinetic interactions are partially predictable based on the available data and can be limited or prevented by a dosage adjustment.
In Vitro Release Kinetics Of The La
We fabricated pills of model DAAs that we could procure mixed inside of a silicone or poly matrix and spray-coated PCL coating around each pill to enable controlled release of the DAA . Silicone and PCL have both been used as drug-release matrices for GRSs and have desirable mechanical properties for use in long-term drug delivery . Additionally, these polymers protect the DAA from degradation in acid. Within 7 d, sofosbuvir degrades by 95% in simulated gastric fluid . However, when mixed with PCL, the drugPCL pill shields the drug particles from degradation in acid . After submersion of drug-polymer pills in SGF for 3 d, PCL protected 89.75% of sofosbuvir, 82.39% of daclatasvir, and 87.11% of ribavirin. Silicone protected 98.96% of ledipasvir.
The DAA in powdered form was mixed homogeneously with either PCL or silicone to form a DAApolymer blend, which was then casted or molded, followed by extraction of individual pills. During the mixing process of drug with PCL or silicone, the drugpolymer mixture was subjected to elevated temperatures. All four DAAs were stable after being subjected to 100 °C for 3 h, confirming that the manufacturing process did not affect drug stability . To prevent a burst release of drug, a layer of PCL was spray-coated on the surface of the pills . Each pill had a height and diameter of 5 mm with a 0.5-mm hole in the center through which to pass the nitinol wire and contribute to the assembled LA-DAAS.
Recommended Reading: What Lab Test For Hepatitis C
Daa Therapy Increases The Risk Of Recurrence In Patients With Previous Hcv
Some of the aforementioned studies merely observed the effect of DAA therapy on HCC recurrence, which ignored the time lag between CR and DAA initiation. Although they were corrected for potential confounding, they still had the pharmacovigilance effect. Researchers who had high hopes for DAA began to calm down and thought about the possible reasons for the high HCC recurrence after DAA therapy. Because DAAs are the accepted standard of care even in patients with previously treated early HCC, it is not feasible or ethical to design randomized controlled trials using an NDAA cohort as the control.
Rehabilitation Of Immune Damage
A great deal of studies have shown that HCV infection will induce the up-regulation of many genes involved in innate immunity characterized by up-regulation of interferon-stimulated genes expression , elevated levels of interferon- sensitive cytokines and chemokines . More importantly, the chronic activation of the innate immune response and the consequent activation of hepatic stellate cells are the initiators of hepatitis and cirrhosis . HCV may interact with immunity response through multiple mechanisms. It is well known that HCV RNA can be recognized by the Toll-like receptor 3 or the RIG-I helicase-mediated pathway in the cytoplasm, resulting in transcriptional activation of type 1 interferon. And type 1 interferon can activate the JAK-STAT signal pathway, which subsequently precedes the transcription of ISGs that have antiviral effects . Simultaneously, the increased type 1 IFN can also trigger natural killer cells and make it a polarized phenotype, elevated cytotoxicity, and down-regulation of the pro- apoptotic factors TRAIL and cytokines .
Unlike interferon-based treatments, DAA treatment acts precisely on some critical steps of HCV replication, thereby preventing HCV replication. It plays a role in the treatment of CHC less dependent on the host’s immune function. Hence, many researchers speculate that the dysfunction of CHC patient’s immune system may be recovered partially or completely with the DAA clearing HCV.
Figure 2
Read Also: What Is Autoimmune Hepatitis C
Treatment After Liver Transplantation
After liver transplantation reinfection of the graft leads to fast development of liver fibrosis, and consequently the risks of organ dysfunction and graft loss are significantly increased . Thus, antiviral treatment is essential to preserve liver function and ensure transplant survival . Severe post-transplantation cholestatic hepatitis and patients with moderate to severe fibrosis need urgent initiation of antiviral treatment to prevent graft loss . In the SOLAR-1 and -2 trials patients after liver transplantation show similar SVR rates compared to nontransplanted patients with the treatment of sofosbuvir/ledipasvir plus RBV . Comparable results were achieved by the combination of sofosbuvir/velpatasvir . These results are supported by data from several real-world studies confirming the efficacy and safety of DAA treatment in the post-transplantation setting. Potential DDI between DAA and immunosuppressive drugs require special attention, in particular when using protease inhibitors. However, the combination of glecaprevir/pibrentasvir is certainly a valuable pangenotypic alternative to sofosbuvir-containing regimens, in particular for patients with impaired kidney function after liver transplantation . During DAA treatment serum levels of immunosuppressant drugs have to be closely monitored.
Study Design And Population
This study is a single-center, observational, prospective study, which enrolled forty consecutive Egyptian patients with concurrent HCV viremia and HCV-related arthritis, who were already assigned to receive the combination of SOF-DCV with or without RBV regimens as well as those who were in the waiting list of the same therapy during the period from June 2017 to December 2017. The study was carried out in both Rheumatology Department and Internal Medicine Department, Faculty of Medicine, University, Egypt.
A total of 40 HCV patients with HCV-related arthritis were included in this study as follows: group I included 20 consecutive patients who were already assigned to receive the combination of sofosbuvir-daclatasvir with or without ribavirin regimens, and group II included 20 consecutive patients who were included in the waiting list for the same therapy.
Informed consents were obtained from all the participants before entering the study. The study was approved by the ethics committee of the university and was conducted in accordance with the declaration of Helsinki.
You May Like: How Can You Contact Hepatitis C
Recommended Monitoring For Patients In Whom Treatment Failed To Achieve A Sustained Virologic Response
RECOMMENDED For patients with cirrhosis, endoscopic surveillance for varices should be performed in accordance with the AASLD guidance on portal hypertension bleeding in cirrhosis. Guidanceb a For, please refer to the appropriate section.b Unlike the AASLD/IDSA HCV guidance, the AASLD guidelines use the GRADE system to rate recommendations please see that document for further information about this rating system.
Ethics Approval And Consent To Participate
Study investigators will ensure this trial is conducted in accordance with the principles of the Declaration of Helsinki and with the ICH Guidelines for Good Clinical Practice .
Ethics approval has been granted by Monash Health Human Research Ethics Committee and Western Australia Aboriginal Health Ethics Committee Planned deviations from this protocol will not occur without approval from the relevant governing bodies. Informed consent will be documented in writing or electronically from all study participants.
Also Check: Treatment To Cure Hepatitis C