Clinical Significance Of Hepatitis C Genotypes
Genotype generally has not been found in epidemiological studies to play a large rolein liver disease progression due to HCV. Rather, genotype is of clinical importanceprincipally as a factor in selecting the appropriate HCV medications for treatment. Please see the HCV Treatment Considerations for more information.
Recommended Reading: Echogenic Liver Consistent With Hepatic Steatosis
Appropriate Uses Of The Hcv Rna Test
There are 4 major reasons that HCV RNA tests are used:
More rarely, HCV RNA is used when either very acute HCV infection is suspected or a false HCV Ab is suspected.
It would not be appropriate to repeatedly order HCV RNA viral load screening for a patient who is not on or was recently on HCV treatment, or to use the HCV viral load to determine the severity of the patient’s infection or the patient’s risk of developing significant liver disease.
Pregnancy And Hepatitis C
Should pregnant women be tested for HCV antibodies?
Yes. All pregnant women should be screened for anti-HCV during each pregnancy, except in settings where the prevalence of HCV infection is < 0.1% . Pregnant women with known risk factors should be tested during each pregnancy, regardless of setting prevalence. Any pregnant women testing positive for anti-HCV should receive a PCR test for HCV RNA to determine current infection status.
Can a mother with hepatitis C infect her infant during birth?
The overall risk of an infected mother transmitting HCV to her infant is approximately 4%8% per pregnancy . Transmission occurs during pregnancy or childbirth, and no prophylaxis is available to protect the newborn from infection. The risk is significantly higher if the mother has a high HCV viral load, or is coinfected with HIV with which the rate of transmission ranges from 8%15% . Most infants infected with HCV at birth have no symptoms.
Should a woman with hepatitis C be advised against breastfeeding?
When should children born to HCV-infected mothers be tested to see if they were infected at birth?
You May Like: What Doctor Treats Hepatitis B
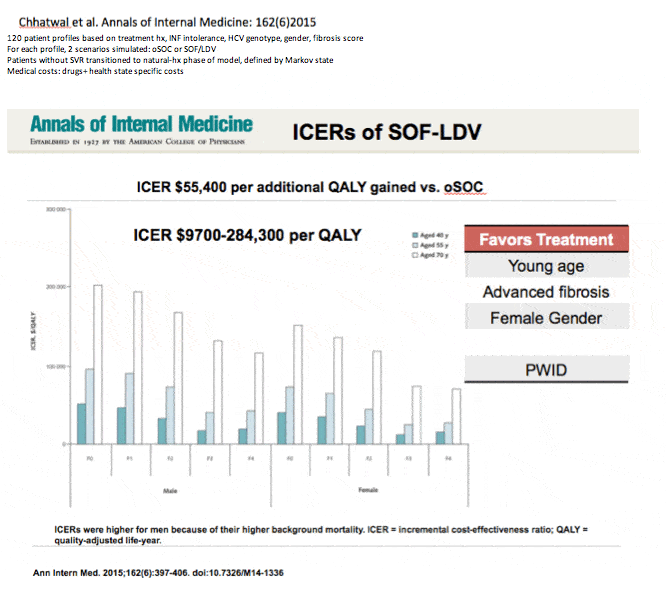
Hcv Treatment Provides Good Value
Despite its cost, HCV treatment provides good value for the money, as expressed in terms of cost-effectiveness. In a recent review, Linas and Nolen note that most studies in the past five years find that HCV treatment falls within the generally accepted value of $100,000 per quality-adjusted life year . They note that these studies do not reflect price decreases that have occurred in the past 1-2 yearsbut the substantial cost burden remains. A critical question is not whether to treat patients with HCV, but when, because of the significant lag time between infection and disease. Facing costs that could overwhelm fixed budgets, many payers have restricted treatments to those with advanced disease or those who are alcohol- or drug-free.
There is some evidence that public payers are relaxing their eligibility restrictions for hepatitis C treatment. Kapadia, Jeng, Schackman, and Bao looked at Medicaid drug utilization data from 2014 to 2016, and found that states that loosened their restrictions had a more rapid increase in prescriptions of direct-acting antivirals than states maintaining their restrictions. The 31 states that implemented Medicaid expansion under the Affordable Care Act saw much more of an increase in utilization than states that did not.
Approach To Choosing Hcv Genotype 2 Treatment Regimen
For patients chronically infected with genotype 2 HCV, two key factors influence the choice and duration of therapy: cirrhosis status and prior treatment experience. In addition, the cost of the regimen, insurance coverage, concurrent medications, and patient and provider preference can play a major role in the regimen choice. The following treatment recommendations are based on the AASLD-IDSA HCV Guidance for initial treatment of adults with HCV genotype 2 and for retreatment of adults in whom prior therapy failed, including those with HCV genotype 2.
You May Like: Can Hiv Lead To Hepatitis
Hepatitis C Viral Rna Quantitative Real
Hepatitis C Viral RNA, Quantitative, Real-Time PCR with Reflex to Genotype LiPA test cost minimal is in Personalabs with price $221.00. Hepatitis C Viral RNA, Quantitative, Real-Time PCR with Reflex to Genotype LiPA test cost max is in SaveOnLabs with price $421.50. This laboratory test is available in 2 online lab test stores.
Factors To Consider Prior To Choosing Initial Treatment Regimen
For initial treatment of persons with chronic HCV genotype 2 infection, three major factors influence the choice of regimen and duration of therapy: the presence or absence of cirrhosis, drug interactions, and medication cost and/or insurance considerations. The treatment regimens for persons with HCV genotype 2 and HIV coinfection are the same as for those for HCV genotype 2 monoinfection, with the following exceptions: persons with HIV-HCV coinfection and compensated cirrhosis should receive a 12-week course of glecaprevir-pibrentasvir versus an 8-week course with HCV monoinfection and additional drug interactions between DAAs and antiretroviral medications need to be taken into consideration.
Also Check: Could I Have Hepatitis And Not Know It
The New Generation Anti
Zepatier is FDA approved in May 2015 which is containing Elbasvir and Grazoprevir recommended for HCV genotypes including 1a, 1b as well as 4 without cirrhosis . According to the literatures, it has been revealed that initiation of treatment with this drug has SVR in 9497% of cases against genotype 1 and 97100% of cases against genotype 4. Moreover, the total of SVR for hepatitis without cirrhotic was 97% while it was 95.7% for cirrhotic . Mavyret is a FDA approved drug from 2017 which is recommended against wide range of HCV genotypes 26 without cirrhosis or with mild cirrhosis . In clinical trials, Mavyret was associated with SVR 92100% for HCV genotype 2 . Daclatasvir plus Sofosbuvir was appropriate for HCV genotypes 2 and 3 patients with SVR 94100% in different human trials . In a cohort study in Egypt, it has been shown that Daclatasvir plus Sofosbuvir dad achieved to SVR 96% for HCV genotype 4 infection after 12 months of treatment . Harvoni is as a RNA polymerase inhibitor which is the first FDA approved hepatitis therapeutic regimen free of interferon and Ribavirin . As noted it can improve the SVR rate from 94 to 99% against genotypes 1, 4, 5 and 6 . In the Fig. 1, some of effective anti HCV drugs and their effects on different compartments of HCV virus has been noted.
Also Check: How To Get Tested For Hepatitis C
Why The High Costs
At this time, theres a short list of blockbuster HCV drugs. Because the FDA only recently approved these drugs, the companies that manufacture them have market exclusivity. That means only these companies can promote and sell the drugs. It also means there are no generic versions of these drugs yet. Generics are typically much cheaper than brand-name versions.
The FDA determines how long this period of exclusivity will last. During this time, the pharmaceutical companies have a lot of freedom in establishing prices. And those who developed the new HCV drugs have set the pricing bar high.
The table below highlights the average cost of treatment for the combination DAAs currently available. Most of these drugs take at least 12 weeks to cure HCV, while the most recently approved drug, Mavyret, can take only eight weeks.
| Generic name |
| $94,800 |
These costs are averages derived from information provided by www.goodrx.com. They were current at the time this article was published.
Read Also: How To Catch Hepatitis C
You May Like: Hepatitis B Vaccine Newborn Dosage
Genotypes: Predictors Of Success
Not everybody with hepatitis C necessarily goes directly to therapy, Nolte points out. This can be a sort of benign infection, and it could be 10 years before someone requires therapy. But if the physician makes the decision to treat a patient, both the viral load and genotype can predict response to therapy. For example, high viral load and genotype 1 are predictors of poor response.
Of the six HCV genotypes, types 1, 2, and 3 are most prevalent in the United States. Type 1 infections respond differently than types 2 or 3 to the current combination therapy of pegylated interferon, an immune modulator, and ribavirin, an antiviral agent. According to Shiffman, a 12-month course of therapy succeeds in about 45 percent of patients infected with type 1, the most difficult to eradicate. Types 2 and 3 are much more sensitive to therapy and can be eradicated in 70 to 90 percent of patients with 4 to 6 months of therapy.
The goal of hepatitis C therapy is to eradicate the virus so it can no longer be detected by a sensitive HCV RNA test. Sustained virologic response is achieved if the HCV becomes undetectable during therapy and remains undetectable six months after the end of therapy. But current treatment algorithms are being challenged as new data become available.
Criteria To Distinguish A New Case From An Existing Case
All jurisdictions are encouraged to track negative HCV viral detection tests to document both spontaneous clearance of infection or sustained viral response to HCV treatment. Cases that have evidence of having cleared the infection at time of initial report or are considered false positive should not be reported to CDC.
If evidence indicating resolution of infection is received after a confirmed chronic case has been reported to CDC, the case report does not need to be modified as it was a confirmed case at the time of initial report. However, negative HCV viral detection test results received on confirmed chronic cases, subsequent to an initial positive result, should be appended to case reports, as feasible, and considered for the purpose of data analysis by each jurisdiction.
For probable chronic cases, the presence of a negative HCV viral detection test result, in the absence of criteria that would allow for confirmation, indicates that a case should not be classified as probable chronic and should not be reported to CDC.
A new chronic case is a newly reported case that does not have evidence of being an acute case of HCV infection. A confirmed acute case may be classified as a confirmed chronic case if a positive HCV viral detection test is reported one year or longer after acute case onset. A confirmed acute case may not be reported as a probable chronic case . For purposes of incidence and prevalence calculations, confirmed chronic HCV cases should be counted.
Recommended Reading: How Can A Person Get Hepatitis C
Enzyme Immunoassays For Detection Of Hepatitis C Antibody
The HCV Ab test is used for initial screening for hepatitis C. The test is performed by enzyme immunoassays , which detect the presence of hepatitis C antibodies in serum. The result of the test is reported as positive or negative. Third-generation EIAs have a sensitivity/specificity of approximately 99%. However, the presence of HCV Ab does not indicate whether the infection is acute, chronic, or resolved. A positive antibody test result should be followed up with an HCV RNA test to confirm that viremia is present.
Tracking The Costs Of Hepatitis C Treatment
Researchers are continuing to create medications that shorten the duration of treatment for hepatitis C.
According to the Pharmacy Times, the cost of treatment can be as low as $54,600 for the 12-week course and the entry to the market of new, cheaper drugs is likely to continue to bring the cost of hepatitis C treatments down.
The level of insurance cover for hepatitis C treatments can vary, depending on a persons insurance policy and overall health.
Some insurance companies will pay for people whose hepatitis C has not responded to less-expensive treatments or for those who are already showing signs of liver damage.
Some insurance companies may require a person to prove they have been drug- and alcohol-free before authorizing treatment.
Insurance companies may believe people who fall into these categories will cost them less money.
If a person has a hepatitis C diagnosis, they may first ask what treatments their doctor recommends. Then, they should contact their insurance company to find out what medications their insurance plan may cover.
Even if an insurance plan does not provide cover for treatments, there are still some patient assistance programs that help reduce the costs of specific treatments.
To find out about these, people can try researching the following:
Obtaining additional financial assistance and discounts can sometimes be a time-consuming and frustrating process.
Read Also: Vaccine Available For Hepatitis B
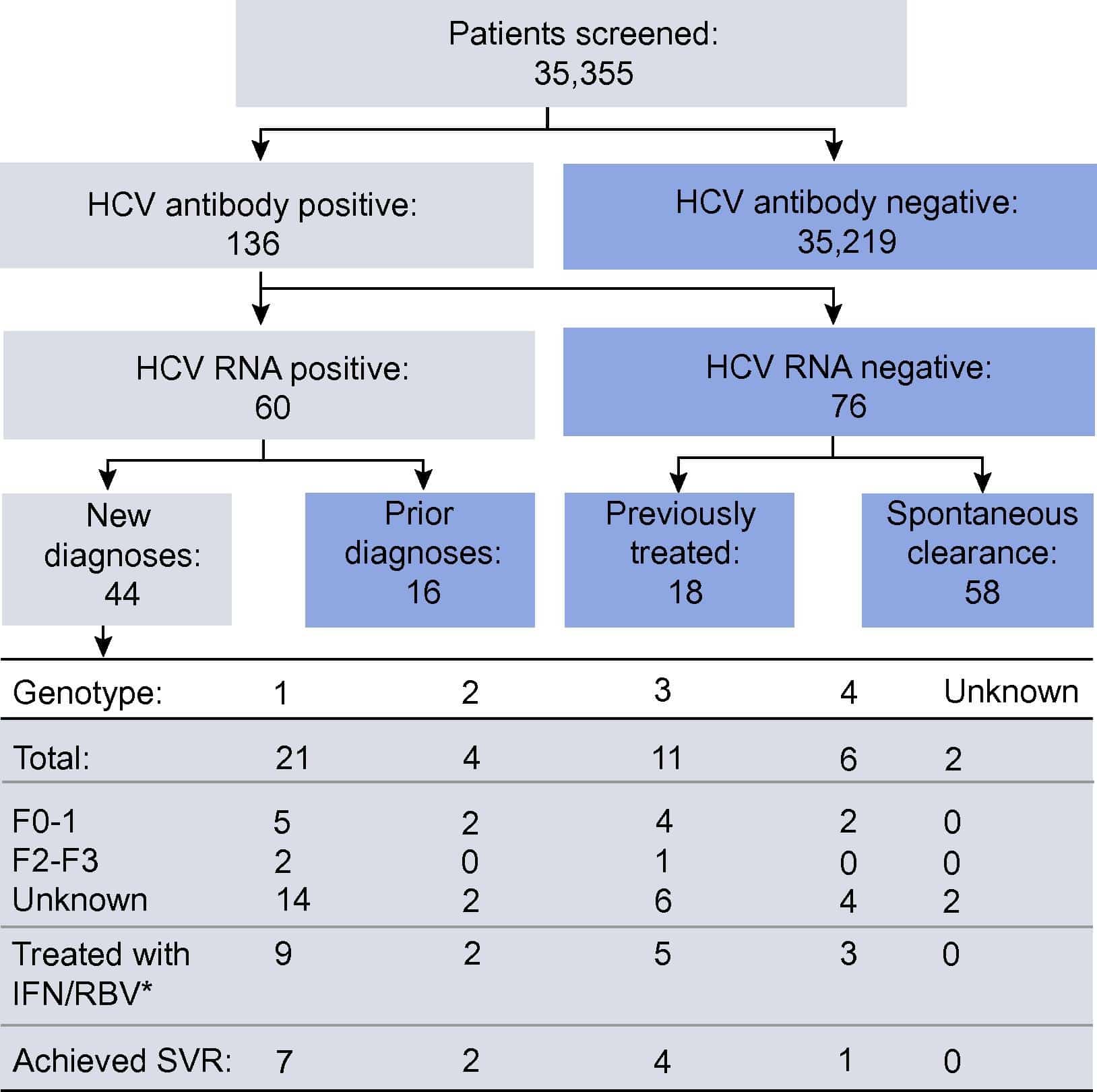
Whats The Optimal Hcv Screening Strategy
Using a simulation model, Barocas, Tasillo, Eftekhari Yazdi, et al. compared the clinical costs, outcomes, and cost-effectiveness of four HCV testing strategies: the existing one one-time testing for adults aged 40 or higher, 30 or higher, or 18 or higher. All strategies included targeted testing of people at higher risk, such as those who inject drugs. As shown below, expanded age-based strategies increased identification and lifetime cure rates.
Figure 3. HCV continuum of care over a lifetime, by strategy
The authors estimate that the existing strategy would identify 71% of all HCV-infected people, and 44% of them would be cured over a lifetime. Compared to existing screening, a strategy of age 18 or older would result in 256,000 additional people identified, 28,000 additional cures, and 4,400 fewer cases of hepatocellular liver cancer over the lifetime of this age group. For people born outside the baby boomer cohort, case detection rates would increase from 74% to 85%, and cure rates would increase from 49% to 61%. Overall, this would represent a 21% reduction in liver-attributable mortality, and an increase in life expectancy from 67.2 to 68.2 years among the affected population.
You May Like: What Medicine Cures Hepatitis C
Are Test Results Accurate
Although no test is perfect, hepatitis C testing is an important and accepted method of testing for HCV. In order to reduce the risk of inaccurate results, doctors take steps to verify a patients diagnosis. For example, a positive test result for hepatitis C antibody requires confirmation with HCV RNA testing.
Don’t Miss: How Did I Get Hepatitis B
Hepatitis C Virus Genotypes
An important variable for all patients with chronic hepatitis C virus is thegenotype of HCV with which they are infected. This is the strain of the virus towhich they were exposed when they were infected, often many years prior to theirevaluation, and it is determined by a simple blood test. Genotypes of HCV aregenetically distinct groups of the virus that have arisen during its evolution. Approximately 75% of Americans with HCV have genotype 1 of the virus, and 20-25% have genotypes 2 or 3, with small numbers ofpatients infected with genotypes 4, 5, or 6. Most patients with HCVare found to have only one principal genotype, rather than multiple genotypes. Genotype 4 is much more common in Africathan in many other parts of the world, genotype 6 is common in Southeast Asia, andeach area of the world has its own distribution of genotypes.
Dont Miss: Royal Canin Feline Hepatic Diet
Study Design And Population
This nationwide cohort study included patients infected with a nonepidemic HCV genotype treated with an interferon-free DAA regimen. Nonepidemic HCV genotypes were defined as genotypes and subtypes other than 1a/1b/2a/2b/3a/4a/4d. All laboratories performing HCV genotyping in the Netherlands were approached. All but 1 participated in the study: the Amsterdam University Medical Centers Sanquin Diagnostics, Amsterdam UMC Groningen, Groningen LUMC, Leiden Erasmus Medical Center, Rotterdam and Maastricht UMC, Maastricht.
Also Check: Hepatitis B Vaccine Schedule For Adults
Hepatitis C Drugs Are Pricey
Antiviral drugs for hepatitis C are very effective, but they come at a steep cost. Just one Sovaldi pill costs $1,000. A full 12-week course of treatment with this drug costs $84,000.
The price of other hepatitis C drugs is also high:
- Harvoni costs $94,500 for a 12-week treatment
- Mavyret costs $39,600 for a 12-week treatment
- Zepatier costs $54,600 for a 12-week treatment
- Technivie costs $76,653 for a 12-week treatment
Hepatitis C drugs are expensive due to the large demand for them, and the high cost of bringing them to market. Developing a new drug, testing it in clinical trials, and marketing it can run pharmaceutical companies nearly $900 million.
Another factor adding to the high cost is the lack of a national health care system to negotiate medication costs on behalf of consumers. Theres also little competition from other drug companies. As a result, hepatitis C drug manufacturers can essentially charge whatever they want.
Prices could drop in the future as more pharmaceutical companies get into the hepatitis C drug market. The introduction of generic versions of these drugs should help drive costs down.
Preparation Prior To Transport
Centrifuge if using SST. Serum or plasma must be removed from the clot within 6 hours of collection. Place specimen in a biohazard bag and seal. Specimens should be stored frozen and shipped to PHOL on ice packs or dry ice.
Upon separation plasma/serum may be stored in secondary tubes for up to 3 days at 2°C to 8°C or at -70°C or colder for longer term.
You May Like: Liver Transplant For Hepatitis B
Understanding Of Lab Tests Results
Please visit the page about hepatitis C on the site associated with The American Association for Clinical Chemistry for better understanding of tests. There you will find the most detailed and full information regarding lab tests. In “common questions” tab you will find answers on the most common questions.
In addition, you can use a special form to ask the question. It is useful, if there is no answer on your question on the web site. A laboratory scientist will answer your question. It is a part of voluntary service provided by the American Society for Clinical Laboratory Science.