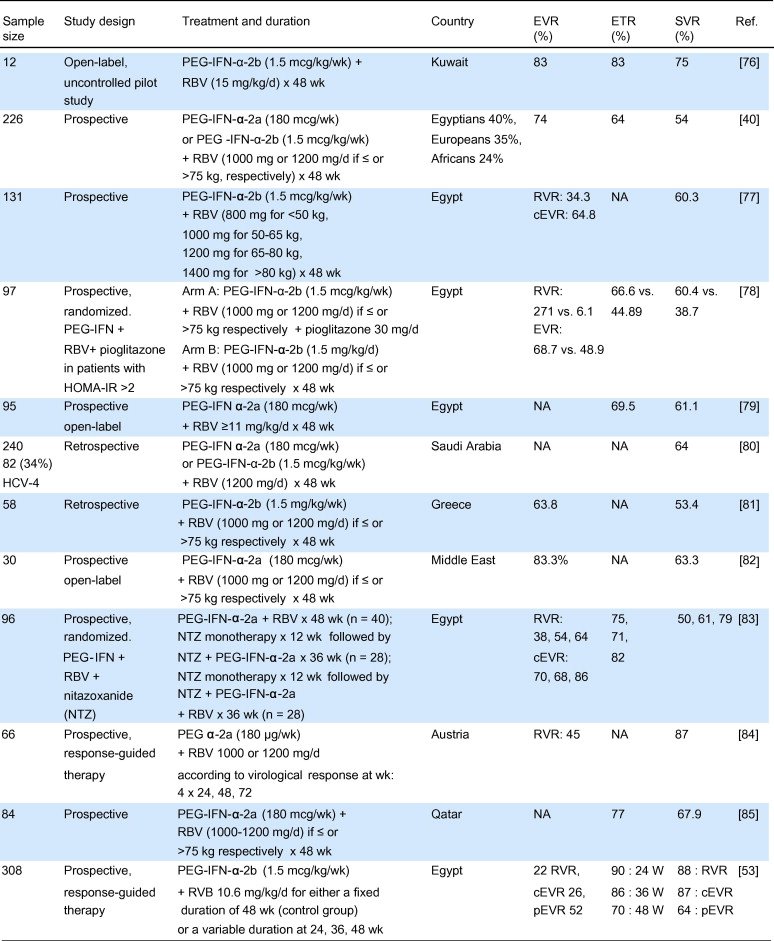
Whats The Difference Between Hepatitis C Genotypes
As mentioned, the different HCV genotypes and subtypes have different distributions throughout the world.
Genotype 1 is the most common HCV genotype in the United States. Its found in nearly 75 percent of all HCV infections in the country.
Most of the remaining people in the United States with HCV infection carry genotypes 2 or 3.
The HCV genotype isnt absolutely related to the rate of liver damage, or the likelihood of eventually developing cirrhosis. However, it can help predict the outcome of treatment.
The genotype can help predict the outcome of anti-HCV therapy with interferon-based treatment regimens. Genotype has also helped to determine treatment.
In some formulations, the recommended doses of ribavirin and pegylated interferon are for people with specific HCV genotypes.
Outcome Of Acute Hcv Infection
After initial exposure to HCV, the infection fails to resolve in the majority of patients who become chronically infected. The ability to evolve into chronic disease associated with liver damage is by far the most striking feature of HCV. The spontaneous clearance of HCV following acute infection in a small proportion of patients has been the focus of intense investigations. It has been proposed that differences in the host cellular or humoral immune responses to HCV are important in spontaneous clearance, but these proposals remain unproved.
Amoroso et al. specifically investigated the role of HCV genotypes in persistence of HCV infection following an acute exposure. The rate of evolution to chronicity after acute exposure to HCV was 92% in patients exposed to HCV genotype 1b infection, compared with 33% to 50% in patients exposed to other genotypes. These data provided evidence that viral factors, including the HCV genotype, may potentially play an important role in the development of chronic infection following acute exposure to HCV.
Clinical Relevance Of Hcv Genotypes
Although the impact of HCV heterogeneity and genotypes on the day-to-day clinical management of chronic HCV infection has not been established, its role as an epidemiologic marker has been clearly shown. Furthermore, the sensitivity and specificity of serologic and virologic assays for the detection of HCV may be influenced by the heterogeneity of HCV. However, the exact role of genotypes in the progression of liver disease, the outcome of HCV infection, and the response to interferon therapy are much less well understood than their role as an epidemiologic marker. The study of these issues has been hampered by the long natural history of HCV infection and the lack of information about the exact time of exposure to the infection. The following subsections in this section specifically address these issues on the basis of the information available.
Recommended Reading: New Medicine To Treat Hepatitis C
Rev Col Gastroenterol Vol29 No3 Bogot Sept 2014
Case Report and Literature Review: Shortened 16 Week Treatment of Patient with Genotype 2 Hepatitis C Infection
Rolando J. Ortega Q. MD. , Mario Moscote G. MD. , Moisés Diago M. MD.
Internist, Gastroenterologist, Hepatologist, Director of the Division of Hepatology and Adjunct Director of the Division of Gastroenterology at the Clínica General del Norte in Barranquilla, Colombia. Institute for Biomedical Research in the Faculty of Health Sciences at Universidad de San Buenaventura in Cartagena, Colombia. E-mail:
Internist, Gastroenterologist and Director of the Division of Gastroenterology Division Director at the Clínica General del Norte. Barranquilla, Colombia.
Internist, Gastroenterologist and Hepatologist. Chief of the Digestive Pathologies Division and Director of the Hepatology Service at the Manager Hepatology Hospital General de Valencia in Valencia, Spain.. E-mail:
Received: 10-03-14 Accepted: 21-07-14
Abstract
Keywords
Hepatitis C, genotype 2, shortened dual therapy, rapid viral response.
INTRODUCTION
CLINICAL CASE
DISCUSSION
There is evidence that suggests that treatment of hepatitis is advisable because it is associated with improvements in patient quality of life, fibrosis, portal pressure, the risk of hepatocellular carcinoma, liver failure and mortality rates .
Acknowledgement
We would like to acknowledge and thank Angie Lopez, an outpatient nurse in the Hepatology department of the Clinica General del Norte in Barranquilla.
REFERENCES
Comparison Of Amino Acid Variation Between The Svr And Non
Next, we compared amino acid variations that were unique, relative to a population consensus, to either the SVR or non-SVR patients for the complete HCV polyprotein and each HCV protein. The number of amino acid variations in the sequences from the SVR patients was significantly higher than in those from the non-SVR patients, when the entire HCV polyprotein was analyzed . These differences were especially significant in E1, p7 and NS5A . This result demonstrated that HCV sequences from patients with SVR comprised a heterogeneous population, while HCV sequences from patients with non-SVR comprised a rather homogeneous population, indicating the existence of unique non-responsive HCV sequences in those regions in E1, p7, and NS5A.
Also Check: When Was Hepatitis C Discovered
Dna Sequencing Clinical Isolates At Two Different Regions Of The Hcv Genome Produces Mostly Concordant Results
Over a 12 month period, the Molecular Diagnostic lab at the University of Wisconsin Hospital and Clinics performed a bidirectional consensus sequencing reaction of 203 base pairs of the 5′ UTR as well as a separate bidirectional consensus sequencing reaction of the 222 base pair fragment of NS5B for 133 consecutive patients. As expected, based on genotype 1 being the most common in the United States, almost 70% of the time the NS5B subtype was 1a . Therefore, if a patient in our population was exposed to multiple HCV strains both the first and the second exposure might be subtype 1a viruses. In all cases but one, the genotype determined by the 5′ UTR matched that determined by the NS5B fragment ,2a), while the corresponding NS5B sequence from that isolate matched best with a genotype 1a sequence .2b). Neither the 5′ UTR 2b sequence nor the NS5B sequence perfectly matched with previously determined sequences in our patient population during this study period, although the 2b sequence was identical to an isolate from one patient prior to this twelve month period of time which had been identified as a 2b 5′ UTR .2a). Sequencing of the isolate with the near identical 2b 5′ UTR sequence by NS5B primers was consistent with a 2b genotype. Furthermore the uridine position at base pair 204 of the 5′ UTR from this isolate was found to be a mixed population containing both uridine as well as the cytidine more commonly seen among isolates characterized as 2b at our institution.
Hepatitis C Genotype 2 Treatment
In the United States, genotype 2 accounts for approximately 13 to 15% of all hepatitis C infections. Given the historically relatively high sustained virologic response rates with the treatment of genotype 2, the data regarding retreatment of patients with genotype 2 in whom prior therapy failed is somewhat limited. The following discussion regarding initial treatment and retreatment of patients with genotype 2 chronic hepatitis C assumes the patient and their clinician have already made the decision to proceed with hepatitis C therapy. The FDA approval of the newest, highly effective, well-tolerated direct-acting antiviral agents has been complicated by the high price of these new agents. For the regimens included as preferred or alternative in the 2014 AASLD/IDSA/IAS-USA Guidance for genotype 2 infection, the cost of the treatment regimens range from approximately $85,000 to $113,000 . Although company-related drug assistance programs provides free medication to some low-income patients, getting medications paid for remains problematic for many clinicians and patients.
Also Check: What Is Hepatitis C Screening Test
Understanding Mutations Of Hepatitis C Genotypes
In addition to the different hepatitis C genotypes and subtypes, there are also quasispecies of the virus. After HCV infects the liver, the virus is constantly reproducing or making copies of itself. This happens on an almost unimaginable scale, with trillions of individual viruses replicating every day. As viruses replicate, some of the copies they make contain errors or mutations in their genetic code. Sometimes, a mutation results in a quasispecies of HCV that is successful at evading the bodys immune system. The immune system is constantly trying to catch up with the HCV virus as it produces slightly different versions of itself. Once the body has successfully eradicated an HCV quasispecies, another takes its place. Experts believe that this process of mutation and quasispecies development is the reason why so many people who are infected with HCV develop chronic disease and why infection is so difficult to cure. This also may be why it is so difficult to develop a vaccine against HCV.3
Treatment Of Hcv Genotype 3 Infection In Non
The combination of SOF plus RBV for 24 weeks was the first interferon-free therapy for patients with HCV genotype 3 infection approved by the FDA. International guidelines differ regarding the recommendations for this regimen. EASL guidelines do not recommend this therapeutic regimen for treatment-experienced cirrhotic patients. On the other hand, AASLD recommends SOF plus RBV as an alternative regimen for patients without cirrhosis with previous PegIFN/RBV failure or treatment-naive patients who are IFN-ineligible .
In naive non-cirrhotic patients with HCV genotype 3 infection, SOF plus RBV for 12 weeks resulted in an overall SVR of 61-68%. However, extending the treatment to 24 weeks led to an approximate 30% increase in SVR rates, ranging from 90 to 96% .
Two large clinical trials evaluated the efficacy of SOF plus RBV for 12 weeks in naive, non-cirrhotic patients infected with HCV genotype 3. The Fission trial included 145 patients, but only 89 achieved SVR . The Positron trial included 84 naive patients who were interferon-ineligible or intolerant, of which 57 reached SVR . The Boson clinical trial found higher SVR among naive and non-cirrhotic patients treated with SOF plus RBV for 16 weeks. Among 70 patients treated, 58 achieved SVR . Another arm of this study evaluated 72 naive non-cirrhotic patients treated for 24 weeks, with an overall SVR of 90% . SOF plus RBV for 24 weeks was also used in the Valence trial, which included 92 patients and 87 achieved SVR .
Recommended Reading: How Serious Is Hepatitis A
The History The Hepatitis C Virus
Hepatitis C virus is an infectious pathogen that causes damage to the liver. HCV was first discovered in 1989 it was simply known as non-A and non-B Hepatitis. It is the most common cause of chronic liver disease ending in liver cirrhosis and hepatocellular carcinoma. Globally, it is a significant cause of death and morbidity affecting about 180 million individuals around the the world, in every nation.
Hepatitis C virus is a family of viruses, similar enough to be called Hepatitis C virus, yet different enough to be classified into subgroups, or breeds. We call these Hep C genotypes.
You might like to think of them as different breeds of dogs.
The Hepatitis C virus is so small it can only be measured in nanometres, one virus particle is about one fifty billionth of a metre .
Consequently, because the virus can not actually be seen, a better way to understand the terms HCV genotypes and subtypes is to compare them to things that we can more readily relate to.
Hepatitis C Genotypes
If we think of dogs we have at one end of the scale are wolves and bull mastiffs and at the other end of the scale we have poodles and terriers. So imagine these as being HCVs genotypes. They are all dogs but have different characteristics and different things will happen if one attacks you. They will all do some damage if they bite you but a wolf will do more damage than a poodle.
1a, 1b, 1c
Genotype and treatment
For example, Harvoni is specifically designed to kill genotype 1.
Geographic Distribution Of Hcv Genotypes
At least six major genotypes of HCV, each comprising multiple subtypes, have been identified worldwide . Substantial regional differences appear to exist in the distribution of HCV genotypes .2). Although HCV genotypes 1, 2, and 3 appear to have a worldwide distribution, their relative prevalence varies from one geographic area to another .2).
Worldwide geographic distribution of HCV genotypes and subtypes. Others indicate unclassified sequences.
HCV subtypes 1a and 1b are the most common genotypes in the United States 3) . These subtypes also are predominant in Europe . In Japan, subtype 1b is responsible for up to 73% of cases of HCV infection . Although HCV subtypes 2a and 2b are relatively common in North America, Europe, and Japan, subtype 2c is found commonly in northern Italy. HCV genotype 3a is particularly prevalent in intravenous drug abusers in Europe and the United States . HCV genotype 4 appears to be prevalent in North Africa and the Middle East , and genotypes 5 and 6 seem to be confined to South Africa and Hong Kong, respectively . HCV genotypes 7, 8, and 9 have been identified only in Vietnamese patients , and genotypes 10 and 11 were identified in patients from Indonesia . There has been disagreement about the number of genotypes into which HCV isolates should be classified. Investigators have proposed that genotypes 7 through 11 should be regarded as variants of the same group and classified as a single genotype, type 6 .
Recommended Reading: What Is Chronic Hepatitis C Without Hepatic Coma
Sofosbuvir Plus Pegylated Interferon/ribavirin
The combination of SOF plus PegIFN/RBV for 12 weeks is the only interferon based therapy recommended by the EASL and AASLD guidelines for the treatment of HCV genotype 3 infection .
In naive non-cirrhotic patients, SOF plus PegIFN/RBV for 12 weeks resulted in an overall SVR of 92-100% . However, efficacy data is scarce: few patients were included in clinical trials and only three studies evaluated the SVR rates in this population. The phase II study included 25 naive non-cirrhotic patients treated with SOF plus PegIFN/RBV for 12 weeks, reaching an overall SVR rate of 92%, but no SVR data according to specific genotype is available 70033-1.). Another phase II study included 17 patients treated with SOF plus PegIFN/RBV for either 12 or 8 weeks and the overall SVR rate was 100% in both arms . The Boson phase III study included 71 naive non-cirrhotic patients with HCV genotype 3 infection treated with SOF plus PegIFN/RBV for 12 weeks, achieving an overall SVR rate of 96% .
In non-cirrhotic patients, including naive and with previous failure to PegIFN/RBV, SOF plus PegIFN/RBV for 12 weeks resulted in high SVR rates . It must be noted that non-significant differences in SVR rates were observed among naive and treatment-experienced patients, but these data need to be cautiously analyzed, since only small cohorts were included in the studies.
Epidemiology Of Hepatitis C Virus Genotypes And Genotypic Variations Around The World
HCV is divided into six distinct genotypes throughout the world with multiple subtypes in each genotype class. A genotype is a classification of HCV virus based on the genetic material in the RNA strands .
Figure 1.1.3. Genotypes of hepatitis C virus.
Genotypes 1, 2, and 3 have a worldwide distribution . Types HCV G1a and G1b predominate in Europe, North and South America, and Japan, accounting for about 60% of global infections. HCV G2 is common in Japan and China and is found in the United States and in northern, western, and southern Europe . HCV G3 is prevalent in Southeast Asia, India, and Australia and is variably distributed in Europe and the United States . HCV G4 is principally found in the Middle East, Egypt, and central Africa . HCV G5 is almost restricted to South Africa , and HCV G6 is found in specific regions in Asia .
Figure 1.1.4. Geographical distribution of hepatitis C virus genotypes.
Osi Obadahn, Sanaa M. Kamal, in, 2018
Don’t Miss: Common Symptoms Of Hepatitis C
Guided Treatment Of Hepatitis C Virus
The appropriate duration of treatment for HCV infection varies depending on the HCV strain that infects the patient. HCV genotypes 2 and 3 respond much better to standard treatment regimens. Thus, only 24 weeks of therapy are needed to achieve maximum benefit, compared to 48 weeks in persons infected with other HCV genotypes. In current clinical practice, treatment is offered to all patients with HCV infection except those with decompensated cirrhosis, where treatment may lead to worsening of the patients condition. Once treatment is initiated, the most reliable means to determine efficacy is to evaluate the response by measuring HCV RNA. Successful treatment is associated with at least two different phases of viral clearance. The first phase, which occurs rapidly over the course of days, is thought to reflect HCV RNA clearance from a circulating pool through the antiviral effect of interferon. In the second phase of clearance, infected liver cells undergo cell turnover and are replaced by uninfected cells. The second phase of clearance is more variable in duration. First-phase clearance is less specific for detecting success of antiviral treatment therefore, it is necessary to evaluate whether second-phase clearance has occurred.
Ahmed Abdel Aziz, in, 2018
Why Does It Matter That I Have Genotype 2
Knowing that you have genotype 2 offers important information about your treatment options and how likely they are to be effective.
Based on the genotype, doctors can narrow down which treatments are most likely to be effective and how long you should take them. This can prevent you from wasting time on the wrong therapy or taking medications longer than you have to.
Some genotypes respond differently to treatment than others. And how long you need to take medicine can differ based on your genotype.
However, the genotype cant tell doctors how quickly the condition will progress, how severe your symptoms might get, or if an acute infection will become chronic.
15 to 25 percent of people clear the hepatitis C infection without any treatment. Since there isnt a way of knowing who falls into this category, in an acute infection, your doctor will recommend waiting for 6 months to treat the virus, since it may clear spontaneously.
Hepatitis C is treated with antiviral drugs that clear your body of the virus and prevent or lessen damage to your liver. Often, youll take a combination of two antiviral drugs for 8 weeks or longer.
Theres a good chance youll have a sustained virologic response to oral drug therapy. In other words, its highly curable. The SVR rate for many of the new hepatitis C drug combinations is as high as 99 percent.
When choosing drugs and deciding how long you should take them, your doctor will usually consider the following factors:
Read Also: How Do You Know If You Have Alcoholic Hepatitis