C Patients Who Have Achieved Hbsag Loss Spontaneously Or With Therapy
Spontaneous HBsAg loss has been reported to occur at the rate of roughly 1% per year, but this rare event does not occur at a linear rate., In a study of 1,076 patients with CHB in Taiwan, cumulative probabilities of spontaneous HBsAg loss were 8.1% after 10 years and increased to 44.7% after 25 years. HBsAg loss can also occur in response to antiviral therapy, being more common with IFN than with NAs. Although progression of liver disease to cirrhosis or hepatic decompensation generally stops when patients lose HBsAg unless other causes of liver injury are present , the risk of HCC persists, particularly if HBsAg loss occurred in patients older than 50 years or in those with cirrhosis or coinfection with HCV or hepatitis D virus ., – Loss of HBsAg with acquisition of anti-HBs has been termed functional cure. This is distinguished from true cure, in which HBsAg and cccDNA are eliminated.
Guidance Statements for Monitoring Patients With Chronic HBV Infection Who Are Not Currently on Treatment
Benefits Of Antiviral Therapy In Patients With Normal Alt
It has long been recognized that timely and effective antiviral treatment can significantly reduce the risk of end-stage liver disease and related death events in CHB patients. In the past, patients with normal ALT level in the clinical immune tolerance period have not advocated antiviral therapy, because they are worried that after antiviral therapy, not only the viral DNA is not effectively suppressed, but it may induce HBV drug resistance mutations . In addition, many experts believe that for HBV-infected people with normal ALT in the immune tolerance phase, the virus and the host are in a state of mutual balance, and the virus generally will not cause obvious damage to the host liver. Moreover, whether direct antiviral therapy in patients with immune tolerance will affect the spontaneous immune clearance remains unknown, and the economic burden of patients also increase. Therefore, they infer that these patients may not benefit significantly from antiviral treatment. However, by establishing Markov model, Kim et al. found that compared with delaying antiviral treatment to active hepatitis stage, the former can reduce the risk of cirrhosis and HCC . Whether or not the patients with immune tolerance are supported to receive antiviral therapy at this stage and no matter what stage of the natural history of CHB patients, antiviral therapy can delay the progress of the disease, improve the survival time and quality of life.
The Function Of Alt And The Origin Of Its Normal Range
Serum ALT is a kind of intracellular functional enzyme catalyzing amino transfer reaction, which is mainly distributed in the liver. It is one of the most sensitive indicators to measure liver function and reflect liver damage . In normal condition, as long as a small amount of ALT is released into the blood, the activity and activity of the enzyme in the serum can be significantly increased. The concentration of ALT in hepatocytes was 1,000~3,000 times higher than that in serum. As long as 1% of hepatocytes are necrotic and the activity of enzymes in blood is doubled, thus serum ALT is a sensitive marker of acute hepatocyte injury .
In fact, serum ALT detection equipment and reagents are different in different countries and regions, thus the results of normal range are not the same . The standardization of ALT normal range needs to be solved urgently. And the individualized ALT reference level may be also a new direction in the future.
You May Like: Royal Canin Hepatic Dog Food Canned
American Association For The Study Of Liver Diseases Recommendations
The 2016 AASLD guidelines for the treatment of chronic hepatitis B as well as select recommendations from the 2018 AASLD guidance update on the prevention, diagnosis, and treatment of chronic hepatitis B are outlined below and in the Guidelines section.
Adults with immune-active chronic hepatitis B infection
Administer antiviral therapy to lower the risk of morbidity and mortality associated with chronic hepatitis B infection.
The recommended initial agent for adults is PEG-IFN, entecavir, or tenofovir.
Adults with immune-tolerant chronic hepatitis B infection
Antiviral therapy is not recommended.
The AASLD suggests obtaining ALT levels at least every 6 months to monitor for potential transition to immune-active or -inactive chronic hepatitis B.
For select patients older than 40 years, the AASLD suggests antiviral therapy in the setting of normal ALT levels, elevated HBV DNA , and significant necroinflammation or fibrosis on liver biopsy specimens.
Adults with HBeAg-positive immune-active chronic hepatitis B who seroconvert to anti-HBe on nucleoside analog therapy
After a period of treatment consolidation , consider discontinuing NA therapy in noncirrhotic HBeAg-positive adults who seroconvert to anti-HBe while on NA treatment. If antiviral therapy is stopped, monitor the patient every 3 months for a minimum of 1 year for recurrent viremia, ALT flares, seroreversion, and clinical decompensation.
Adults with HBeAg-negative immune-active chronic HBV infection
Inpatient care
Relationship Between Alt And Liver Injury
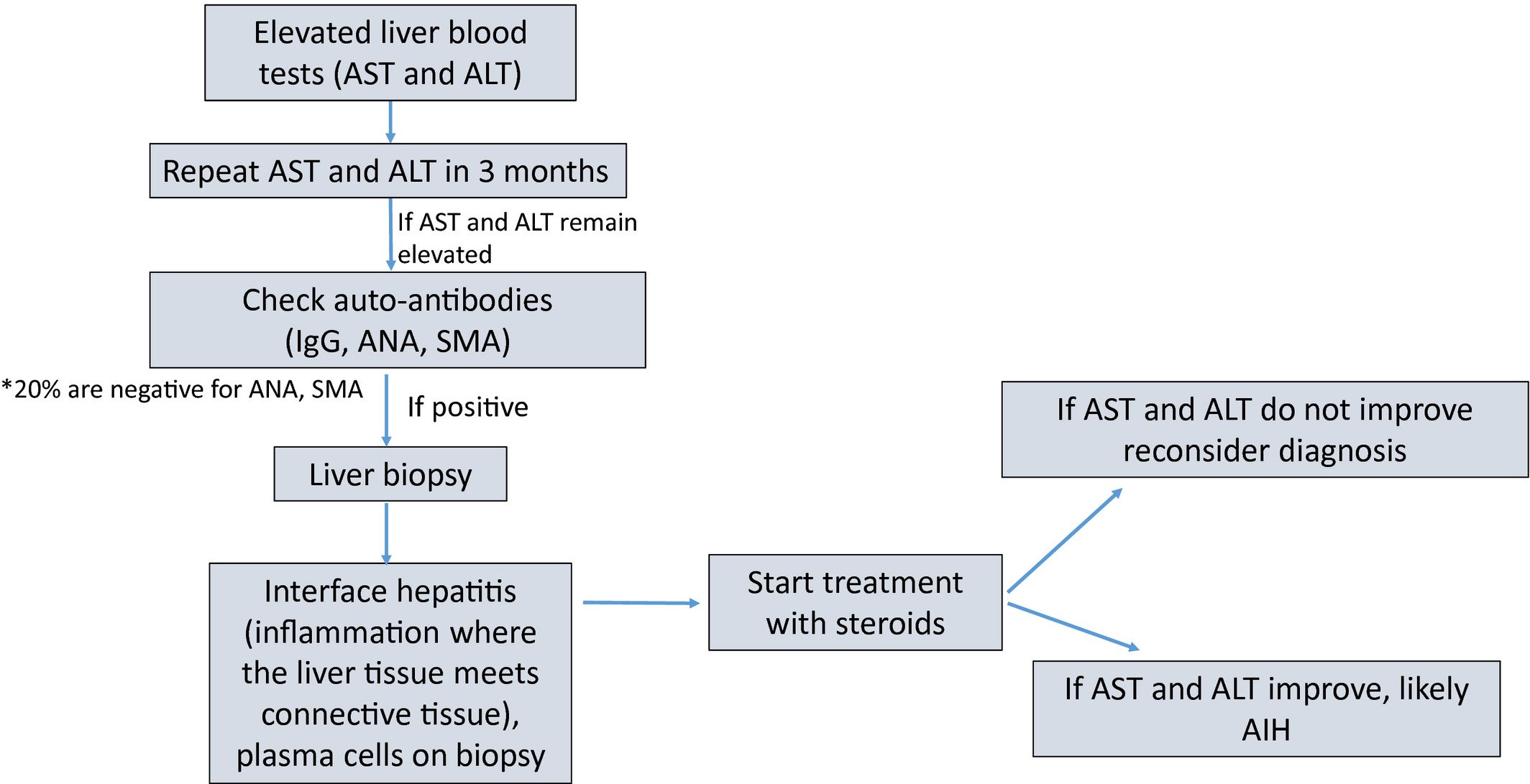
The elevation of serum ALT level in different degrees indicates that there is inflammatory reaction in liver tissue. In the recovery period of the liver disease, with the gradual disappearance of inflammation, ALT levels will gradually return to normal. CHB is a chronic infectious disease which is mediated by HBV and infiltrated by a variety of inflammatory cells in the liver. For example, CTL induces apoptosis of target cells by secreting perforin and expressing FasL Neutrophils and monocytes in the liver produce reactive oxygen species, carbon monoxide, and reactive nitrogen species, which mediate the injury of infected hepatocytes and the formation of local inflammatory injury and reaction with monocytes/macrophages infiltration. Generally, ALT in peripheral blood of patients with HBV infection leading to liver inflammatory injury will increase in varying degrees, but there are also some patients whose ALT in peripheral blood is still in the normal range or only slightly increased even when liver histology has shown more significant inflammation or fibrosis.
Read Also: What Is The Treatment For Hepatitis
World Health Organization Recommendations
The 2015 WHO guidelines for the prevention, care, and treatment of persons with chronic hepatitis B infection indicates treatment priority for individuals of all ages who have chronic hepatitis B infection and clinical evidence of compensated/decompensated cirrhosis , regardless of their levels of ALT or HBV DNA, or their HBeAg status.
Treatment is recommended for adults with chronic hepatitis B infection without clinical evidence of cirrhosis , but who have all of the following features , and regardless of HBeAg status :
- Are older than 30 years
- Have persistently abnormal ALT levels
- Have evidence of high-level HBV replication .
In individuals with HBV/human immunodeficiency virus coinfection, the AASLD recommends initiating ART in all those with evidence of severe chronic liver disease, regardless of CD4 count, as well as those with a CD4 count of 500 cells/mm3 or below, regardless of their liver disease stage.
However, the AASLD does not recommend antiviral therapy, indicating it can be deferred, in individuals with all of the following , regardless of HBeAg status or age :
- No clinical evidence of cirrhosis
- Persistently normal ALT levels
- Low levels of HBV DNA replication . ]
I Nonliver Solid Organ Transplant Recipients
All patients evaluated for nonliver solid organ transplantation should be tested for HBsAg, anti-HBc, and anti-HBs. Patients who are HBsAg-positive should have ALT and HBV-DNA measurements and undergo staging with biopsy or elastography to determine whether advanced fibrosis or cirrhosis is present. Though previously felt to be a contraindication, in the current era of antiviral therapies, patients with compensated cirrhosis without portal hypertension may be considered for nonhepatic solid organ transplantation, with the largest clinical experience in kidney transplantation. Patients with decompensated cirrhosis and those with compensated cirrhosis and portal hypertension should be considered for combined liver and kidney, heart, and/or lung transplantation.
Compared with nonâHBV-infected recipients, untreated HBsAg-positive nonliver transplant recipients have a higher mortality rate, with liver-related complications as a major cause of death., Antiviral therapy, however, can mitigate this mortality risk., , To effectively prevent reactivation, therapy should begin before or at the time of surgery, regardless of ALT and HBV-DNA status, given that these parameters preceding transplantation have only a limited ability to predict HBV reactivation after transplantation. Entecavir, TDF, and TAF are preferred antivirals because of the low rate of resistance with long-term use.
Guidance Statements for Management of Hepatitis B in Nonliver Solid Organ Transplant Recipients
Don’t Miss: Hepatitis C Antibody Blood Test
C Counseling Of Hbsag
All pregnant women should be screened for HBsAg. Pregnant women with CHB should be encouraged to discuss with their obstetrician and/or pediatrician the prevention of mother-to-child transmission. Hepatitis B immune globulin and HBV vaccine should be administered to their newborn < 12 hours after delivery., Antiviral therapy in the third trimester is recommended for pregnant women with serum HBV DNA > 200,000 IU/mL.,
A proportion of women have hepatitis flares with or without HBeAg seroconversion within the first months after delivery. Seroconversion rates of up to 17% have been described. It has been postulated that the rapid decrease in cortisol levels characteristic of the postpartum state is analogous to the steroid withdrawal therapy that has been used to elicit seroconversion. Although the flares are often mild and resolve spontaneously, cases of acute liver failure have been described in the peripartum period.- Extending third trimester antiviral therapy from 2 to 12 weeks postpartum did not protect against postpartum flares in one study, supporting the AASLD guideline recommendation that antiviral therapy given for prevention of mother-to-child transmission be discontinued at the time of delivery or up to 4 weeks postpartum.
Although antiviral drug labels do not recommend breastfeeding when taking these drugs, clinical studies support the safety of these drugs during breastfeeding.,
Guidance Statements on Counseling of Women in Pregnancy
What Is Immune Tolerance
Generally, immune tolerance is defined as the specific non-response state of the immune system after receiving specific antigen. The liver has a unique immune regulation function, which can promote the tolerance to HBV, and this may be the main reason of HBV persistence and chronic infection. In 1972, Dudley et al. firstly proposed that HBV persistent infection was related to immune tolerance, and the liver injury was determined by T cell-mediated immune response . In 1983, Liaw et al. reported that HBeAg clearance was related to the enhancement of host immune response . Based on the above findings, Chu et al. firstly divided the natural history of HBV infection into three phases of immune tolerance, immune clearance, and residual integration , and then divided it into four phases of immune tolerance, immune clearance, inactive carrier status and reactivation . The latter four phases are the current widely used classification of hepatitis B natural history.
Also Check: What Is The Treatment For Hepatitis B
How To Define The Immune Tolerance Phase Of Hbv Infection
The immune tolerance phase of HBV infection has been defined and described in many international CHB management guidelines . Although these guidelines have some differences in the definition of immune tolerance phase of HBV infection, they also have common characteristics, such as positive HBsAg , positive HBeAg, high level of HBV-DNA , persistently normal ALT , as well as no obvious inflammation, necrosis and fibrosis in liver pathology.
At present, the inconsistent definition of immune tolerance phase in different guidelines is mainly manifested in the level of serum HBV DNA and ALT. Because of the controversy in serum HBV DNA and ALT levels, clinicians must pay attention to following details when judging a chronic HBV infection whether in the immune tolerance phase. Firstly, the guidelines require that patients’ serum ALT level is persistently normal, rather than a certain cross-sectional serum ALT within the normal range. Therefore, in clinical practice, we need patients to provide reliable laboratory reports of dynamic serum ALT and comprehensively evaluate various potential factors that may cause ALT level fluctuations. Secondly, on the premise of meeting other conditions, the higher the serum HBV-DNA level, the more likely the patient will be in the immune tolerance period. Because only the very high serum HBV DNA level can accurately indicate the peaceful coexistence of the virus and the host.
Chb With Normal Alt Still Had Obvious Liver Histological Abnormalities
We previously analyzed the pathological results of 141 CHB patients with normal ALT and found that 47.5% of the patients had significant inflammation and 33.3% had significant fibrosis or cirrhosis . Among HBeAg-positive patients with persistently normal ALT, the proportion of patients with significant liver inflammation or fibrosis was 27.8~49.4% . Recently, Prof. Zhuang and his colleagues also reported that 53.2% of HBeAg-negative patients with normal ALT have obvious liver fibrosis . Among them, 44.6% of patients with serum ALT > 20 U/L had obvious hepatic necrotizing inflammation and 61.0% had obvious hepatic fibrosis while only 26.5% of patients with ALT 20 U/L had obvious hepatic necrotizing inflammation and 41.7% had obvious hepatic fibrosis. These results confirmed that a considerable number of HBeAg-negative CHB patients with normal ALT had obvious liver histopathological changes, and even 46.2% of the patients with low HBV DNA level had obvious liver fibrosis. A long-term follow-up study of 1,965 untreated inactive carrier HBeAg-negative patients in Taiwan found that during an average follow-up of 11.5 years, 16% progressed to reactivation and 3% developed cirrhosis . Therefore, patients with normal ALT in inactive carrier status may also have significant liver pathological changes and high risk of liver cancer or death .
Don’t Miss: Hepatitis C And Liver Cancer
Immunopathogenesis Of Hepatitis Due To Hbv Reactivation
Hepatitis B virus is a hepatotropic virus and after entry into hepatocytes, the HBV nucleocapsid containing partially double-stranded HBV DNA enters the nucleus where the viral polymerase repairs dsDNA into full-length, covalently closed circular , the nuclear reservoir of HBV. Reverse transcription, viral replication, and encapsidation occur in the cytoplasm before either viral assembly and release or recycling of the nascent nucleocapsid into the nucleus to replenish the pool of cccDNA . It is the persistence of these low levels of cccDNA in hepatocytes which are thought to explain the long-term risk of HBVr with potent IST that exists even in individuals who have cleared the HBV infection, with serological clearance of hepatitis B surface antigen . The clinical outcome of HBV infection is highly dependent on a complex interplay between the virus-specific host immune response involving cytotoxic HBV-specific CD8 T cell and natural killer /NK-T cell responses, cytokine-mediated non-cytolytic responses as well as B-cell-mediated humoral immunity . In keeping with this, resolution of HBV infection with loss of HBsAg with or without the development of anti-HBs has been demonstrated to require CD4 helper T cells for the efficient development of virus-specific adaptive CD8 T cell responses and B-cell antibody production .
Fig. 1
Aasld Guidelines For Treatment Of Chronic Hepatitis B

Norah A. Terrault
University of California San Francisco, San Francisco, CA
Norah A. Terrault
University of California San Francisco, San Francisco, CA
Potential conflict of interest: Dr. Jonas consults and received grants from Gilead. She received grants from Bristol-Myers Squibb and Roche. Dr. Chang advises Genentech, Alnylam, and Arbutus. Dr. Terrault consults for Bristol-Myers Squibb and received grants from Gilead. Dr. Bzowej received grants from Gilead, Synageva, and Ocera.
The funding for the development of this Practice Guideline was provided by the American Association for the Study of Liver Diseases.
This Practice Guideline was approved by the AASLD on August 1, 2015.
-
- American Association for the Study of Liver Diseases
-
- ALT
-
- Grading of Recommendation Assessment, Development and Evaluation
-
- HBV
Don’t Miss: Hepatitis B Shot Side Effects
Clinical Guidelines For Children With Chronic Hepatitis B
In general, the clinical guidelines for children are the same as for adults – visits are usually scheduled every six months or once a year. Most children do not need drug treatment, but they still need to be monitored regularly to make sure they remain healthy and to detect any problems with their liver as soon as possible. Visits will include a physical exam, blood tests, and possibly an imaging study of the liver .
AASLD guidelines provide guidance for treating children under the Updated Recommendations on the Treatment of Patients With Chronic Hepatitis B, section 9A.
The Hepatitis B Foundation convened the first Pediatric HBV Workshop and invited the nations leading pediatric liver specialists to develop the first national recommendations for children living with hepatitis B to ensure that they receive the best care possible. These recommendations have been published in highly respected, peer-reviewed journals and provide expert guidance for the care of infected children.
Hepatitis B Foundations Clinical Guidelines for Pediatric HBV
HBF’s Pediatric HBV Screening and Monitoring Recommendations Published in Pediatrics in November 2009Haber BA, Block JM, Jonas MM, Karpen SJ, London WT, McMahon BJ, Murray KF, Narkewicz MR, Rosenthal P, Schwarz KB. Recommendations for screening, monitoring, and referral of pediatric chronic hepatitis B. Pediatrics 124:e1007-13.
Recommendations For The Initial Evaluation Of Hbsag
All patients
References
Centers for Disease Control and Prevention. Hepatitis B information for health professionals: hepatitis B FAQs for health professionals. http://www.cdc.gov/hepatitis/HBV/index.htm. Available at . Updated May 31, 2015 Accessed: May 23, 2017.
Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009 Jan 20. 150:104-10. . .
McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001 Nov 6. 135:759-68. .
Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997 Jun 26. 336:1855-9. .
Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995 Jan. 21:77-82. .
Yu MC, Yuan JM, Ross RK, Govindarajan S. Presence of antibodies to the hepatitis B surface antigen is associated with an excess risk for hepatocellular carcinoma among non-Asians in Los Angeles County, California. Hepatology. 1997 Jan. 25:226-8. .
Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977 Jul 1. 197:17-25. .
Read Also: How To Test For Hepatitis C Virus