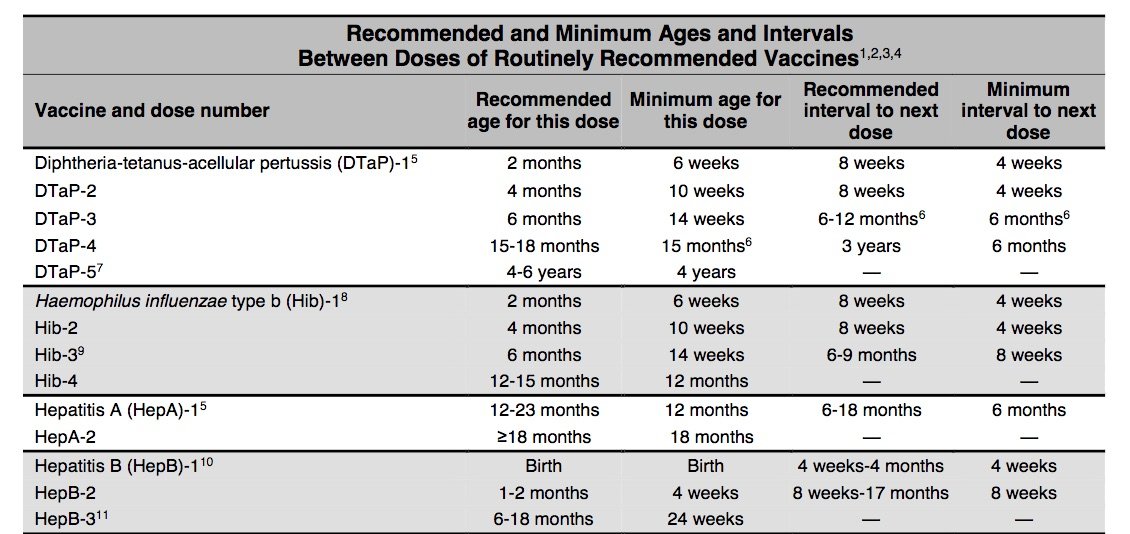
Dosage Forms & Strengths
Contraindicated
- belimumab
belimumab decreases effects of hepatitis b vaccine by immunosuppressive effects risk of infection. Contraindicated. Do not administer live vaccines 30 days before or concurrently with belimumab.
Serious – Use Alternative
Monitor Closely
Minor
-
chloroquine decreases effects of hepatitis b vaccine by pharmacodynamic antagonism. Minor/Significance Unknown.
- ozanimod
ozanimod decreases effects of hepatitis b vaccine by immunosuppressive effects risk of infection. Minor/Significance Unknown. No clinical data are available on the efficacy and safety of vaccinations in patients taking ozanimod. Vaccinations may be less effective if coadministered with ozanimod.
Usual Pediatric Dose For Exposure To Hepatitis B Virus
Nabi-HB, prophylaxis of infants born to HBsAg positive mothers:
Nabi-HB, postexposure prophylaxis:HepaGam B, Postexposure prophylaxis:
- If hepatitis B vaccine is refused or in known vaccine non-responders, administer a second dose one month after the first dose.
Household exposure to person with acute HBV infection:
- Infants under 12 months old: 0.5 mL intramuscularly administer concurrently with hepatitis B vaccine.
- Treatment of other household members is not recommended without an identifiable blood exposure , then treat like sexual exposures.
Do The Benefits Of The Hepatitis B Vaccine Outweigh Its Risks
Every year in the United States about 2,000 people die following an overwhelming hepatitis B virus infection. In addition, every year about 22,000 people are infected with hepatitis B. Some of them will remain chronically infected, putting them at high risk of the long-term consequences of hepatitis B virus infection: cirrhosis and liver cancer. In fact, with the exception of influenza and COVID-19 viruses, hepatitis B virus causes more severe disease and death in the United States than any other vaccine-preventable disease. On the other hand, the hepatitis B vaccine is an extremely rare cause of a severe allergic reaction called anaphylaxis. To date, no one has died from this reaction, but it is theoretically possible that this could occur.
Because hepatitis B virus is a common cause of severe disease and death in the United States, and because the hepatitis B vaccine does not cause permanent damage or death, the benefits of the hepatitis B vaccine clearly outweigh its risks.
You May Like: First Signs Of Hepatitis C
Hepatitis B Vaccine On The Nhs
A hepatitis B-containing vaccine is provided for all babies born in the UK on or after 1 August 2017. This is given as part of the 6-in-1 vaccine.
Hospitals, GP surgeries and sexual health or GUM clinics usually provide the hepatitis B vaccination free of charge for anyone at risk of infection.
GPs are not obliged to provide the hepatitis B vaccine on the NHS if you’re not thought to be at risk.
GPs may charge for the hepatitis B vaccine if you want it as a travel vaccine, or they may refer you to a travel clinic for a private vaccination. The current cost of the vaccine is around £50 a dose.
Babies And Children Can Develop Chronic Hbv
You may be wondering why the recommendations for the HBV vaccine start on the first day of life.
Adults who contract HBV will likely not experience long-term complications from hepatitis B. But the same is not the case for babies. As many as of babies who contract an HBV infection at birth from their mothers become chronically infected with HBV.
Children between the ages of 1 and 5 who get an HBV infection have a 25 percent of people who become chronically infected during childhood will develop liver cancer or cirrhosis. Thats why pediatricians want children to have immunity from HBV from the earliest possible age. Many babies and children exposed to HBV receive post-exposure prophylaxis, which decreases chance of infection.
If youre pregnant, youll most likely have a blood test to see if youre positive for hepatitis B. This allows doctors to find out if theres a chance that you could pass on the virus. These tests are highly sensitive and have a good accuracy rate, but they arent perfect. Additionally, a pregnant person may become infected between the time of the test and giving birth. The first dose of the vaccine given at birth lowers the risk of a newborn baby contracting hepatitis B.
Also Check: Signs Of Having Hepatitis C
Hepatitis B Vaccination In Pregnancy
Hepatitis B infection in pregnant women may result in severe disease for the mother and chronic infection for the baby.
This is why the hepatitis B vaccine is recommended for pregnant women who are in a high-risk category.
There’s no evidence of any risk from vaccinating pregnant or breastfeeding women against hepatitis B.
And, as it’s an inactivated vaccine, the risk to the unborn baby is likely to be negligible .
What Do The Results Mean
A hepatitis B blood panel consists of three tests that can be done with just one blood sample:
- Hepatitis B surface antigen . A positive test indicates that youre infected with hepatitis B and that you can spread it to other people. Further tests are needed to see if you have an acute or chronic infection.
- Hepatitis B core antibody . A positive result can indicate a past or current hepatitis B infection, but doesnt mean youre immune. A positive result needs to be interpreted by a doctor by examining the results of the other two tests.
- Hepatitis B surface antibody . A positive test indicates that youre protected from hepatitis B either through previous infection or vaccination .
The combination of these tests can indicate your hepatitis B status and whether you need to be vaccinated. Your test will give a negative or positive result for each category depending on whether your results are above or below the cutoff value.
Most peoples test results fall into the following categories. But its possible to have a result that doesnt fall into one of these groups. If youre reading your results yourself, be careful not to confuse HBsAb with HBcAb.
| HBsAG |
is associated with hepatitis B immunity after vaccination. But research has found that anti-HBs decline over time.
A found that more than 95 percent of people had anti-HBs levels greater than 10IU/L two years after vaccination. But this rate decreased to 70 percent after eight years.
Don’t Miss: What Happens When You Get Hepatitis C
Use In Special Populations
Pregnancy
There are no adequate and well-controlled studies designed to evaluate RECOMBIVAX HB in pregnant women. Available post-approval data do not suggest an increased risk of miscarriage or major birth defects in women who received RECOMBIVAX HB during pregnancy.
Nursing Mothers
Data are not available to assess the effects of RECOMBIVAX HB on the breastfed infant or on milk productions/excretion. The developmental and health benefits of breastfeeding should be considered along with the mothers clinical need for RECOMBIVAX HB and any potential adverse effects on the breastfed child from RECOMBIVAX HB or from the underlying maternal condition.
Pediatric Use
Why Do You Need A Hepatitis B Shot
Hepatitis B is a viral infection that cant be transferred person-to-person unless you have contact with an infected persons bodily fluids. Annual infection rates of HBV are going down in the United States thanks to vaccines. So you might be wondering if you or your child needs a shot to protect against hepatitis B.
Recommended Reading: Do I Have Hepatitis C
For Adults And Children
This vaccine schedule involves three doses within 2 months, followed by a booster dose at 1 year.
The initial accelerated doses provide immediate protection from HBV, and the booster dose helps provide long-term protection.
Below is the accelerated vaccination schedule approved for both adults and children:
| Vaccine series | |
|---|---|
| 2 months after the first dose | 1 year after the first dose |
Recommended Adult Dosing Volume Of Monovalent Hepatitis B Vaccine
- Age 19 years and younger: Use 0.5 mL per dose .
- Age 20 years and older: 1.0 mL per dose .
For a one-page sheet reviewing the hepatitis B dosing schedule for children and adults, consult IACs Hepatitis A and B Vaccines: Be Sure Your Patients Get the Correct Dose. For complete dosing information, consult the ACIP hepatitis B vaccine recommendations for adults.
Don’t Miss: How Does Hepatitis B Affect The Body
Which Adults Should Be Vaccinated Against Hepatitis B
According to CDC recommendations, adults in the following groups are recommended to receive hepatitis B vaccine:
General
- All people age 18 years and younger.
- Anyone 19 years and older who wants to be protected from hepatitis B.
People at risk for infection by sexual exposure
- Sex partners of people who are hepatitis B surface antigen -positive.
- Sexually active people who are not in long-term, mutually monogamous relationships.
- People seeking evaluation or treatment for a sexually transmitted disease.
- Men who have sex with men.
People at risk for infection by percutaneous or permucosal exposure to blood or body fluids
- Current or recent illegal injection drug users.
- Household contacts of people who are HBsAg-positive.
- Residents and staff of facilities for developmentally challenged people.
- Healthcare and public safety workers with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids.
- People with end-stage renal disease, including predialysis, hemo-, peritoneal- and home-dialysis patients.
Others
- International travelers to regions with intermediate or high levels of endemic HBV infection.
- People with chronic liver disease.
- People with HIV infection.
- People with diabetes who are age 19 through 59 years. For those age 60 and older, clinicians should make a determination of need for
- vaccination based on their patients’ situation.
In a future issue, we will review the various hepatitis B serologic tests, who needs testing, and when they need it .
Important Information About Vaccine And Hepatitis B Immunoglobulin Shot Administration
Where available, the hepatitis B birth-dose and HBIG should be administered within 24 hours of birth in order to prevent the transmission of hepatitis B from mother to child. It is very important that the shots be given in opposite limbs, to ensure the highest effectiveness. Please see chart above for more information.
Don’t Miss: How Can You Contact Hepatitis B
Why Should I Vaccinate My Newborn Child If I Know That I Am Not Infected With Hepatitis B Virus
Before the hepatitis B vaccine, every year in the United States about 18,000 children were infected with hepatitis B virus by the time they were 10 years old. This statistic is especially important because people are much more likely to develop liver cancer or cirrhosis if they are infected early in life, rather than later in life .
About 9,000 of the 18,000 children infected in the first 10 years of life caught the virus from their mother during birth. However, many young children didn’t catch the disease from their mother. They caught it from either another family member or someone else who came in contact with the child. Because hepatitis B can be transmitted by relatively casual contact with items contaminated with the blood of an infected person, and because many people who are infected with hepatitis B virus don’t know that they have it, it is virtually impossible to be “careful enough” to avoid this infection.
For these reasons, all young children are recommended to receive the hepatitis B vaccine. The best time to receive the first dose is right after birth. This will ensure that the child will be protected as early as possible from catching hepatitis B from people who dont know that they are infected with the virus.
Listen to Dr. Offit explain why newborns get the hepatitis B vaccine by watching this short video, part of the series Talking About Vaccines with Dr. Paul Offit.
Hepatitis B Vaccination Schedule For Children And Infants
The Centers for Disease Control and Prevention recommends that babies and children receive three 0.5 milliliter doses of either Engerix-B or Recombivax HB, starting just after birth.
The current recommended hepatitis B vaccine schedule for children and infants is as follows:
| Hepatitis B Vaccination Schedule for Infants and Children | |
|---|---|
| Hepatitis B Vaccine Dose | |
| 3 | 618 months old |
If your child is undergoing hemodialysis, your healthcare provider may recommend that they receive additional doses of the HBV vaccine.
You May Like: How Did I Get Hepatitis B
Hepatitis B Vaccination Booster Dose After 18 Years Maintains Long
Primary vaccination against hepatitis B virus at birth may not provide adequate lifelong antibody levels, but a booster vaccine at age 18 years reinforces antibody levels for at least 4 more years, according to a study published in Infectious Diseases.
Vaccination against HBV is recommended in the first year of life to prevent infection, and studies demonstrate that this provides protection for 90% of the population for 30 years. Data on response to booster doses and long-term protection are lacking therefore, researchers sought to understand the protection duration of the HBV vaccine and the effect of additional doses on the level of protection against infection.
The researchers conducted a retrospective analysis of data from January 2013 to December 2016 of healthcare students in Israel. Participants were aged 19 to 25 years. Immunization history was obtained from medical records, including data for receipt of birth-dose HBV vaccine and booster at age 18 years. This booster is common in Israel, as many teenagers undergo emergency medical technician training that requires HBV vaccination regardless of previous immunization. Baseline antibody titer levels were measured at first clinic visit. A participant was considered protected against HBV if their anti-HBs titer was > 10 MIU/mL any participant with titers below this level received a booster dose of the HBV vaccine.
Reference
International Hepatitis B Vaccine Schedules
*Please note that the first dose should be given as soon as possible. Additional doses require minimum time intervals between doses in order for the vaccine to be effective.
The hepatitis B vaccine is an injection that is generally given in the arm and as a three-dose series. The World Health Organization recommends a 0, 1, and 6-month vaccine schedule, though schedules may vary based on a countrys national immunization program. Completing the hepatitis B vaccine series, preferably beginning at birth, will ensure protection against hepatitis B, hepatitis delta and lower the lifetime risk of liver cancer. Greater than 90% of babies and up to 50% of young children who are not vaccinated and are infected with hepatitis B will have lifelong infection, which makes the birth dose essential to their protection. Please note that the vaccine brand name, manufacturer and associated schedules for adults, children and infants may be unique to different countries, though there is a list of WHO prequalified vaccines.
3-Dose Vaccine Series for Infants
The World Health Organization recommends all infants receive the first dose of the hepatitis B vaccine within 24 hours of birth and to complete the vaccine series with additional shots at 1 month and 6 months of age. Beginning the hepatitis B vaccine at birth will ensure protection against hepatitis B for life.
3-Dose Vaccine Series for Children and Adults
4-Dose Combination Vaccine Series for Infants
Additional Resource Links:
Recommended Reading: Hepatitis C Viral Load Quest
Hepatitis B Vaccine: Canadian Immunization Guide
For health professionals
Last partial content update : May 2022
The footnotes in and the accompanying text description for the figure have been revised to align with the corresponding figure in Protocole d’immunisation du Québec, 5e édition from which it was adapted.
Last complete chapter revision :
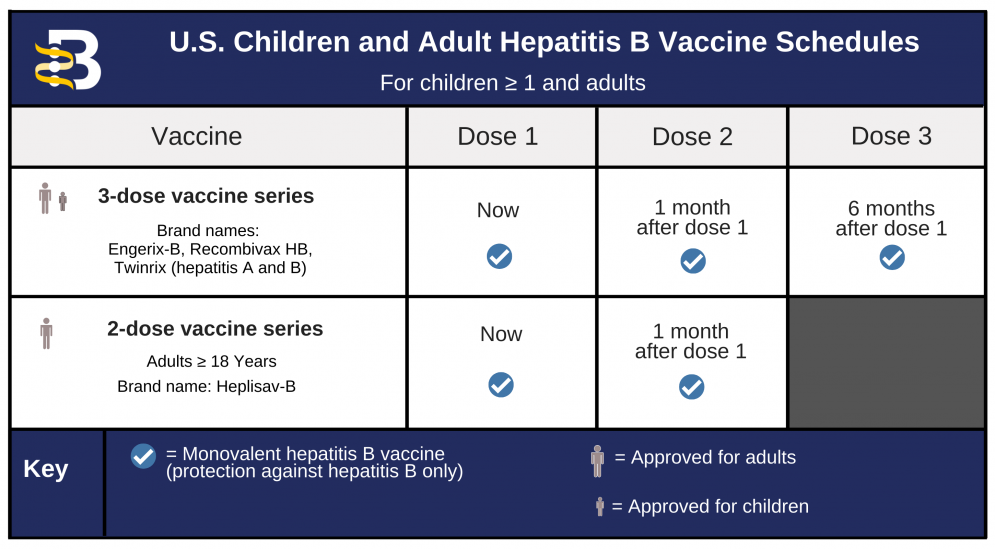
Us Infant Hepatitis B Vaccine Schedules
*Please note that the first dose should be given as soon as possible. Additional doses require minimum time intervals between doses in order for the vaccine to be effective.
3-Dose Vaccine Series for Infants
Since 1991, ALL medically stable infants with a birth weight of at least 2,000 g in the U.S. are recommended to receive the first dose of hepatitis B vaccine within 24 hours of birth. The additional 2 doses are given at 1 month and 6 months of age.
4-Dose Vaccine Combination Series for Infants
Combination vaccines, such as the pentavalent and hexavalent vaccines, include protection against 5 or 6 diseases, including hepatitis B. The first shot is usually given at 6 weeks of age, but in order to protect infants from hepatitis B beginning at birth, a monovalent or single dose of the hepatitis B vaccine is also recommended within 24 hours of birth. The hepatitis B vaccine series can then be completed with the pentavalent or hexavalent vaccine with the recommended schedule.
Also Check: What Does Hepatitis C Do To The Body
How Is The Hepatitis B Vaccine Made
People are protected against hepatitis B virus infection by making an immune response to a protein that sits on the surface of the virus. When hepatitis B virus grows in the liver, an excess amount of this surface protein is made. The hepatitis B vaccine is made by taking the part of the virus that makes surface protein and putting it into yeast cells. The yeast cells then produce many copies of the protein that are subsequently used to make the vaccine. When the surface protein is given to children in the vaccine, their immune systems make an immune response that provides protection against infection with the hepatitis B virus.
The first hepatitis B vaccine was made in the 1980s by taking blood from people infected with hepatitis B virus and separating or purifying the surface protein from the infectious virus. Because blood was used, there was a risk of contaminating the vaccine with other viruses that might be found in blood, such as HIV. Although contamination with HIV was a theoretical risk of the early, blood-derived hepatitis B vaccine, no one ever got HIV from the hepatitis B vaccine. That is because the blood used to make vaccine was submitted to a series of chemical treatments that inactivated any possible contaminating viruses. Today, there is no risk of contaminating the vaccine with other viruses because the surface protein is manufactured in the laboratory.