Specimen Collection And Processing
Ten milliliters of venous blood were taken from each study participant at each site and was dispensed into an ethylene diamine tetra-acetic acid tube and transported on dry ice to Armauer Hansen research institute , where the plasma was separated via centrifugation at 3500 rpm/ 5min, transferred into cryotubes, and stored at – 20 °C until used for HIV, HBV, and HCV serology.
Management Of Pregnant Women With Hiv
Expert consultation is recommended for the management of pregnant women with HIV-HCV coinfection. Current recommended DAA treatments for HCV have limited data for use in pregnancy. Note that ribavirin is absolutely contraindicated for use during any time of pregnancy. Effective combination antiretroviral therapy with at least three drugs is recommended to treat HIV for all pregnant persons with HIV-HCV coinfection, regardless of CD4 cell count or HIV RNA levels. Suppressive antiretroviral therapy for pregnant persons, which markedly lowers the risk of perinatal HIV transmission, may also reduce the risk of perinatal HCV transmission.
Impact Of Hcv Infection On Natural History Of Hiv
Most studies have reported that HCV does not significantly impact HIV disease progression. Some studies have shown that coinfection with HCV may blunt increases in CD4 cell counts after initiation of antiretroviral therapy, whereas others have shown no significant impact of HCV on immune reconstitution. Achieving a sustained virologic response with HCV treatment has not been shown to impact CD4 count or percentage.Chronic HCV infection increases the risk of hepatotoxicity due to antiretroviral therapy in persons with HIV. Nevertheless, for nearly all individuals with HIV-HCV coinfection, including those with cirrhosis, the benefits of antiretroviral therapy outweigh the risks of liver injury caused by antiretroviral medications, particularly with use of currently recommended antiretroviral regimens in the United States, which rarely are associated with hepatotoxicity when compared to older antiretroviral regimens.
Don’t Miss: Can You Get Hepatitis C From Alcohol
Treatment For Genotype 1 Of Hepatitis C
The evidence supports that the beginning of the antiretroviral therapy and, therefore, controlling early replication of HIV and maintaining a good immunological situation are the first measures to adopt in the coinfected patients. Existing data indicate that antiretroviral treatment can slow down the progression of chronic HCV liver disease in the coinfected patient, even in the carriers of liver disease, increasing their survival .
Studies based on liver biopsies have demonstrated a relationship between the management of antiretroviral treatment, immunological improvement, and the presence of lower grades of hepatic fibrosis .
3.2.1 Boceprevir or telaprevir + PR
In patients coinfected with HIV and HCV genotype 1 and without previous treatment for HCV SVR after treatment with Boceprevir or telaprevir was higher than in those treated with PR .
Efficacy and side effects with both triple patterns were similar to those observed in monoinfected patients. The dose of RBV was 800 mg/d in almost all patients. Although significant pharmacokinetic interactions have been described, the co-administration of lopinavir/r, atazanavir/r, and darunavir/r, allowed in the study with PR/BOC, did not affect efficacy .
The results of the Unite 115 study support the dosage of TVR every 12 h in coinfected patients and ART based on IP/r or raltegravir, as well as the possibility of shortening the duration to 24 weeks in patients without cirrhosis with HCV-RNA undetectable in S4 and S12.
Fast Facts About Acute Hepatitis C In 2020

Persons aged 20-39 years had the highest incidence of acute hepatitis C
66% of cases with risk information reported injection drug use
During 2020, rates of acute hepatitis C were highest among males, persons 20-39 years of age, American Indian/Alaska Native persons, those who reported using injection drugs, and those living in the eastern and southeastern states.
You May Like: How Did Hepatitis C Start
Hiv And Hepatitis B And Hepatitis C Coinfection
Hepatitis B and hepatitis C are liver infections caused by a virus. Because these infections can be spread in the same ways as HIV, people with HIV in the United States are often also affected by chronic viral hepatitis.
Viral hepatitis progresses faster and causes more liver-related health problems among people with HIV than among those who do not have HIV. Liver disease, much of which is related to HBV or HCV, is a major cause of non-AIDS-related deaths among people with HIV.
Given the risks of hepatitis B or hepatitis C coinfection to the health of people with HIV, it is important to understand these risks, take steps to prevent infection, know your status, and, if necessary, get medical care from a health care provider who is experienced in treating people who are coinfected with HIV and HBV, or HIV and HCV.
Evaluation Of Alcohol Use
Numerous studies have found a strong association between the use of alcohol and the development of liver fibrosis and hepatocellular carcinoma. Some studies have demonstrated that the risk of developing cirrhosis and decompensated liver disease in persons with chronic HCV infection is 2- to 3-fold higher for individuals with significant alcohol intake compared with those who have minimal or no alcohol intake. The threshold level above which alcohol potentiates the progression of HCV disease is unknown, but it appears that even moderate levels of alcohol consumption accelerate histological lesions in persons with chronic HCV infection. Individuals who are identified as having alcohol use disorder or dependence should be referred to an addiction specialist and/or treatment program.
Don’t Miss: Antiviral Medication For Hepatitis B
Coinfection With Hepatitis B In Patients With Hiv
Hepatitis B, caused by the hepatitis virus B , is an important problem of public health and it is the most serious kind of viral hepatitis. It can cause chronic liver disease and carries a high risk of death for cirrhosis and liver cancer. It is estimated that in the world there are 2 billion people infected with HBV and more than 350 million with chronic liver infection .
Coinfection of HBV with the human immunodeficiency virus is common since they have the same transmission routes.
In Western Europe and in the United States, it has been found that 710% of HIV patients have a chronic infection by HBV, with men who have sex with other men being the group with the highest prevalence . Patients coinfected with HBV and HIV have an increased risk of liver cirrhosis, terminal liver disease and death by hepatic pathology, especially in patients with low CD4 lymphocyte counts and concomitant use of alcohol . There is a greater risk of hepatocarcinoma and adverse hepatotoxic effects in patients using therapy with highly effective antiretroviral drugs coinfected with hepatitis B .
The antigens and antibodies associated with the infection of hepatitis B virus are as follows:
Surface antigen : Associated to active infection. Identify patients who are infectious and can be detected from 3 to 5 weeks after the infection. When it remains more than 6 months high, it is related to chronic infection with HBV .
| Type infection | |
|---|---|
| Chronic hepatitis B mutant pre-core |
Evaluation Of Persons Diagnosed With Hcv Coinfection
Due to the rapidly changing landscape of HCV treatment, the AASLD-IDSA HCV Guidance are regularly updated. These guidelines recommend that persons with current HCV infection should be evaluated by a practitioner who is prepared to provide comprehensive HIV management. A comprehensive evaluation of persons with HIV who are diagnosed with HCV coinfection should include routine laboratory evaluation, HCV-specific tests, status of hepatitis A and B, and assessment of liver fibrosis.
Read Also: Pcr Quantitative Test For Hepatitis C
Treatment For Genotypes 2 And 3 Of Hepatitis C
3.3.1 PEGIFN + RBV
In patients coinfected with HIV/HCV genotypes 2/3, the probability of reaching an SVR with pegIFN-alpha-2a or alpha-2b and adjusted RBV weight for 48 weeks is 6271% .
Patients without cirrhosis who achieve an RVR can be treated for 24 weeks without reduction of response rates . In patients with genotype 2 or 3, without previous treatment, due to the lower efficacy and toxicity associated with prolonged administration, treatment with PR should be considered for exceptional use, when the guidelines considered are not available preferred or alternative:
PR × 48 s . In patients with RVR, PR × 24 s, in the absence of cirrhosis .
3.3.2 SOF + RBV
In four clinical studies, phase III, with monoinfected patients with genotype 2, treated with SOF/RBV for 12 weeks, the SVR ranged between 86 and 97% . Also, in the PHOTON 1 studio in patients without prior treatment coinfected with genotype 2, the treatment with SOF/RBV for 12 weeks showed an RVS12 of 88% in patients pretreated, the SVR after 24 weeks of treatment was 92%.
Although the data are inconclusive, pretreated cirrhotic patients could benefit from a treatment of more than 12 weeks. In the FUSION study, in patients, the SVR was pretreated 60% in cirrhotic patients treated for 12 weeks and 78% in those treated for 16 weeks .
3.3.3 SOF + DCV
Kinetics And Interactions Of Serum Hbv Dna And Hbsag
Theoretically, HCV DAA therapy has no direct effect on the replication of HBV. Unexpectedly, we found that serum HBsAg and HBV DNA kinetics during treatment were found to be inversely correlated . However, no difference in HBsAg kinetics was observed among patients with or without HBV virologic reactivation.
Kinetics of HBsAg during and 48 weeks after end of DAA. A, Mean change in HBV DNA and HBsAg from baseline. P value was determined using Spearman correlation. Spearman correlation factor between HBsAg change and HBV DNA change during treatment. B, HBsAg change in patients with or without HBV reactivation. P value was determined using the Wilcoxon 2-sample test. DAA, direct-acting antiviral HBsAg, hepatitis B surface antigen HBV, hepatitis B virus.
Overall, 45% patients had HBsAg decline 0.5 log10 at least at 1 time point through posttreatment week 48 84% occurred for the first time during the treatment period, and the remaining 16% occurred for the first time during the posttreatment follow-up period. In a multivariate analysis, no factor was identified to be associated with HBsAg decline 0.5 log10 .
One patient developed HBsAg seroconversion. In a multivariate model, low HBsAg level at baseline was associated with HBsAg loss.
You May Like: Can Hepatic Steatosis Be Reversed
Hepatitis A And Hepatitis B Status And Immunization
In persons with chronic HCV infection, superinfection with hepatitis A virus can cause fulminant hepatitis. Thus, all persons with chronic HCV infection should be assessed for immunity to HAV with total hepatitis A antibody and those without immunity to HAV should receive hepatitis A vaccine. All persons with HIV-HCV coinfection should also be screened for HBV by checking hepatitis B surface antigen and hepatitis B surface antibody . Individuals without immunity to HBV should be vaccinated against HBV. Awareness of hepatitis B status in persons with chronic HCV has taken on increased importance with recent reports of HBV reactivation and hepatitis flares during treatment of HCV with direct-acting antiviral agents.
General Approach To Persons With Hiv
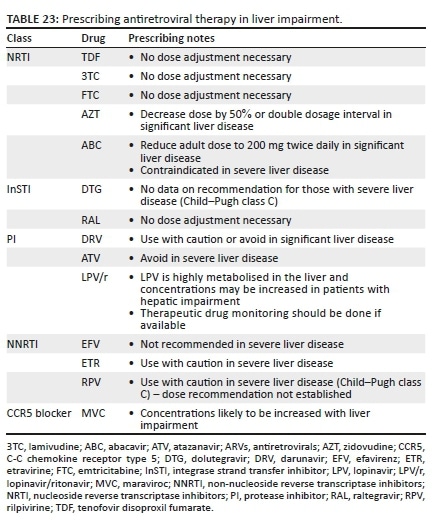
With increasing data showing a number of direct-acting antiviral agents are highly effective and safe for the treatment of HCV in persons with HIV, all persons with HIV-HCV coinfection should be evaluated for treatment of HCV. Rates of HCV cure with DAA-based therapy have uniformly exceeded 95% and experts now consider the approach to treatment of HCV in persons with HIV coinfection similar to that in persons with HCV monoinfection, except for needing to consider drug interactions between DAAs and antiretroviral medications. A proactive and aggressive approach for HCV is needed in persons with HIVidentify and treat HCV in all persons with HIV-HCV coinfection. This strategy would lead to improved health outcomes and longer survival in persons with HIV, as well as reduced transmission of HCV.
Recommended Reading: How Does One Contract Hepatitis
Molecular Hcv Rna Tests
Molecular diagnostic tests for HCV specifically detect HCV RNA and are commonly referred to as a Nucleic Acid Test or Nucleic Acid Amplification Test . The HCV NAT becomes positive approximately 1 to 2 weeks after initial HCV infection. Low HCV RNA levels may be intermittently detectable very early after infection. The NAT test has become the gold standard supplemental test for patients who have positive HCV antibody screening tests. The NAT can usually determine whether a patient with a positive HCV antibody test has chronic HCV or resolved HCV infection. In addition, the NAT can be used to diagnose individuals with acute HCV infection, but unlike with acute HIV, the HCV RNA test can be negative or barely detectable with very acute HCV infection. In a clinical scenario where HCV RNA is negative but acute HCV is highly suspected, it is advisable to repeat the HCV RNA several months later.
Summary And Future Directions
HCV/HBV co-infection is commonly encountered in endemic areas and among individuals at risk of parenterally transmissible infections. How the two viruses interact with each other in the liver is partly understood. In addition to academic interest, these data will help develop more effective antiviral therapy and efficient strategies to prevent HBV reactivation post HCV cure by DAA.
For HCV/HBV co-infected patients, the same genotype-dependent treatment recommendations for single chronic hepatitis C can be applied. The value of DAA-based therapy in co-infected patients has been demonstrated in a recent clinical trial and prolonged follow-up . The long-term benefits of curing HCV by DAAs await further observatory studies. However, HBV reactivation remains a concern to be resolved. Last but not least, for patients with active hepatitis B, more studies are needed to determine the optimal regimen to simultaneously treat both viruses.
Recommended Reading: What Happens To Your Body When You Have Hepatitis C
Frequency Of Reassessment Of Liver Disease
Ongoing assessment of liver disease is recommended for individuals in whom HCV therapy is deferred. Routine HCV-related monitoring for all persons with HIV should include laboratory assessment of hepatic function every 3 to 6 months, at a minimum annual evaluation is appropriate to reevaluate hepatic fibrosis stage and to discuss modifiable risk factors for fibrosis .
What Is Hepatitis C
Hepatitis C is a liver infection caused by the hepatitis C virus . Hepatitis C is a blood-borne virus. Today, most people become infected with HCV by sharing needles or other equipment to inject drugs. For some people, HCV infection is a short-term or acute illness but for more than half of people who become infected with HCV, it becomes a long-term, chronic infection. Chronic HCV infection is a serious disease that can result in long-term health problems, even death. The majority of infected people might not be aware of their infection because they do not have any symptoms. There is no vaccine for hepatitis C. The best way to prevent HCV infection is by avoiding behaviors that can spread the disease, especially injecting drugs.
You May Like: What Causes Hepatitis B And C
Treatment Of Hcv In The Setting Of Hbv Coinfection
All individuals with chronic HCV infection should be tested for HBV infection. Testing should include HBsAg, anti-HBc and anti-HBs serology . Current hepatitis B infection is defined by HBsAg positivity, with chronic hepatitis B infection defined as presence of infection for more than 6 months . All individuals with current HBV infection should be referred for specialist management. Past HBV infection is defined by HBsAg negativity, positive anti-HBc ± positive anti-HBs serology . Occult hepatitis B infection is very rare, but is defined by positive HBV DNA in the absence of HBsAg in most cases, the HBV DNA level is very low anti-HBc is normally positive.
|
Table 7. Definitions of hepatitis B virus infection, by HBV test results |
|
Test |
In the absence of further data at this time, the following conclusions have been drawn about risk of HBV reactivation. There is a risk gradient for the occurrence of HBV reactivation, wherein HBsAg-positive individuals have a moderate risk of HBV reactivation. HBsAg-positive people should have HBV DNA levels measured at baseline and should be considered for antiviral therapy according to current guidelines . If antiviral therapy for HBV is not indicated, active monitoring of ALT and HBV DNA levels should be performed during HCV treatment .
Bookmark
Factors Associated With Hiv And Hiv/hbv Co
Among the risk factors, age , gender , tattoo , marital status , province , clinical status , and chronic consumption of alcoholic beverages significantly associated with the rate of HIV infection . Of the risk factors, only age exhibited a significant association with HIV/HBV co-infection frequency. About 55% and 14% of chronic liver disease patients were chronic consumers of alcohol and cigarette smokers, respectively. Consequently, chronic alcohol consumption and smoking showed a marginal association with HCC than non-HCC cases . Compared with non-HCC, older age exhibited a significant association with HCC cases .
|
Table 3 Factors Associated with Human Immunodeficiency Virus Infection |
|
Figure 2 Trend of HIV and co-HIV/HBV infection across the different age groups of chronic liver disease patients in Ethiopia. |
Don’t Miss: Rx Vitamins For Pets Hepato Support
Risk Of Hepatocellular Carcinoma With Hiv
The incidence rate of hepatocellular carcinoma is 2 to 8% per year in persons with HCV-related cirrhosis in those with HIV-HCV coinfection, the HCC rates appear to be even higher, especially among patients with low CD4 counts. In a large retrospective Veterans Health Administration cohort study, investigators compared the risk of cirrhosis and HCC in persons with HIV monoinfection and HIV-HCV coinfection and demonstrated that HIV-HCV coinfection dramatically promotes the development both of HCC and of cirrhosis .
Baseline Characteristics Of Study Patients
As of December 31, 2019, 79,245 PLWH initiated free ART between 2010 and 2018 in Guangxi, China. Excluding 291 patients under 18 years old, two without follow-up data and 20,713 without HBV and HCV testing results, a total of 58,239 individuals were eligible and included in the analysis . Of those included participants, 12% died, 16% were lost to follow-up, 8% dropped out of treatment and 64% were active on treatment by the end of follow-up.
Flow chart of study sample selection. ART Antiretroviral therapy
The baseline characteristics of the study patients are shown in Table âTable1 1 6,707 participants had HIV-HBV co-infection, 3,828 had HIV-HCV co-infection, 857 and had triple infection. Two fifths of patients were over 50 years old 68.3% were male and 63.7% were married. The majority of patients were infected through heterosexual intercourse, followed by homosexual intercourse , intravenous drug use and other causes . Prior to ART initiation, 59.8% of the patients had CD4 countsâ¤350 cells/mm3, and 5.9% of the patients were classified as WHO clinical stage III or IV. Patients with initial ART regimens of stavudine -based, azidothymidine -based, tenofovir disoproxil fumarate -based and lopinavir-ritonavir -based accounted for 8.5%, 33.7%, 46.8% and 10.1% of all patients, respectively. Most patients used first-line ART regimens, 21.3% used second-line regimens, and 43.1% used TDF-based ART regimens for more than 2 years.
Recommended Reading: Causes Of Hepatitis C Virus