California Pays Insurers Millions More For Hepatitis C Drugs
Both drugs are produced by Gilead, and their price has dropped some, as competitors have hit the market, but Higginbotham still hasn’t been able to get the cure.
Gilead isn’t the only company that produces expensive drugs. Prices for prescription medications for various conditions have been rising faster than inflation for years.
President Donald Trump says the pharmaceutical industry is “getting away with murder,” and he’s vowed to do something about it.
So what could he do in the case of hepatitis C medications?
One solution that’s been proposed is to buy the company. Specifically buy Gilead Sciences Inc.
Dr. Peter Bach, the director of health policy and outcomes at Memorial Sloan Kettering Cancer Center in New York, says the U.S. government could save money and treat everyone in the nation who has hepatitis C if it bought Gilead Sciences, rather than just buying Gilead’s products.
Hepatitis C kills more Americans than any other infectious disease, according to the Centers for Disease Control and Prevention, and it leads to a liver transplant more often than any other condition.
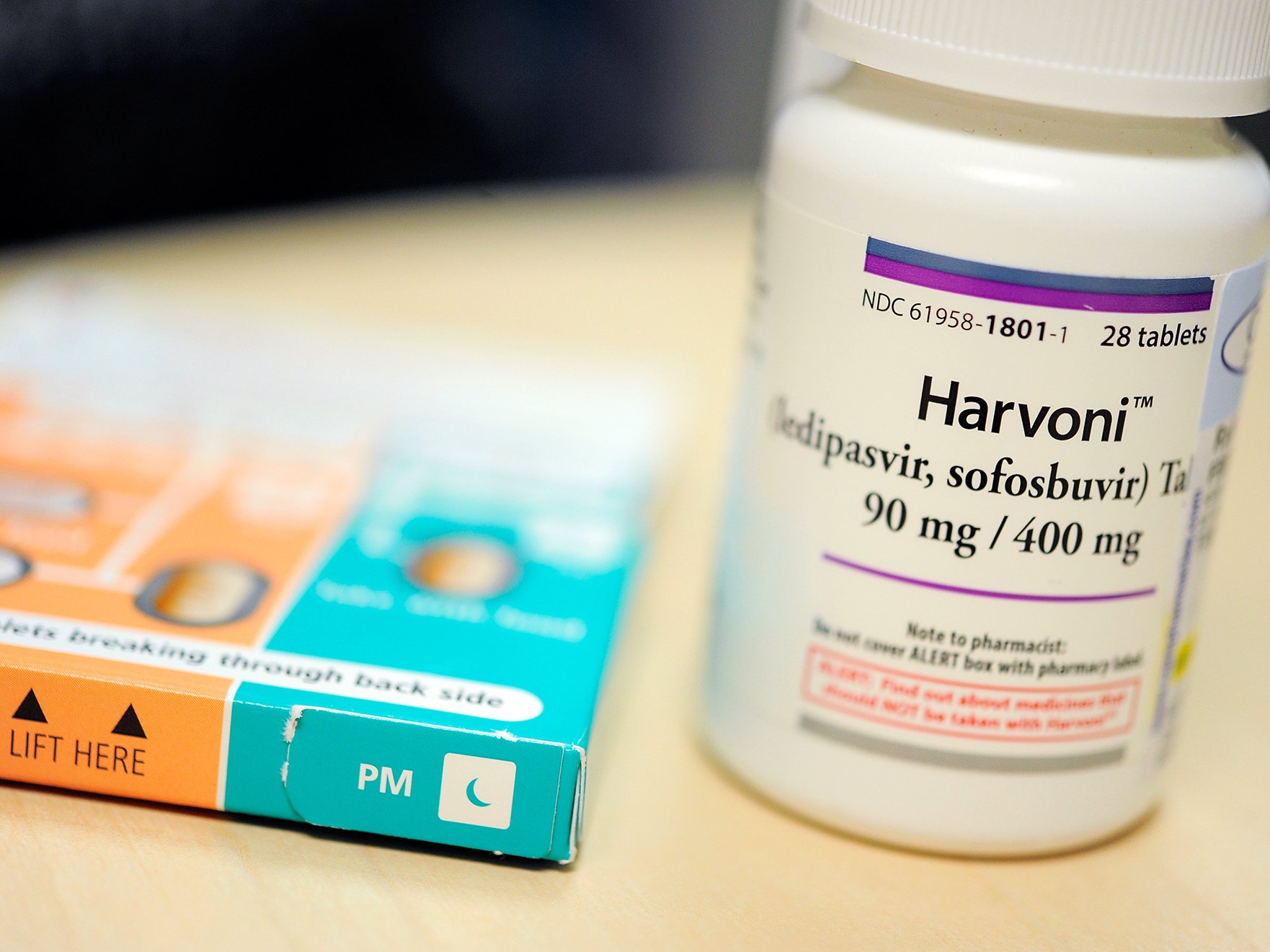
A year after Gilead got FDA approval for Sovaldi, it launched Harvoni, a combination medicine that includes Sovaldi’s key ingredient and a second medication that attacks the hepatitis C virus in a different way. Sovaldi and Harvoni are the first drugs to consistently eradicate the virus, which has infected an estimated 2.7 to 3.3 million people in the U.S.
toggle caption
Gilead Hcv Treatment News
It was reported by officials of Gilead Sciences, Inc. in a recent press release that they were going to create a new subsidiary, Asequa Therapeutics LLC. Officials stated they were planning to use Asequea Therapeutics to launch generic versions of Gileads leading treatments for chronic hepatitis C virus in the United States: sofosbuvir 400mg/ velpatasvir 100mg and ledipasvir 90mg/ sofosbuvir 400mg . These two drugs are amongst a list of current FDA-approved drugs for HCV treatment.
A Perspective From Our Ceo: Gilead Subsidiary To Launch Authorized Generics To Treat Hcv
Foster City, Calif., September 24, 2018 Today, Gilead announced a plan to introduce a generic version of our leading cures for hepatitis C in the United States more than a decade before the expiration of the patents.
We made this unusual decision because we believe that it is the fastest way to lower list prices for our HCV cures without significant disruption to the healthcare system and our business, as a bridge to longer term solutions aimed at reducing patients out-of-pocket medication costs, improving access for Medicaid patients and providing greater pricing transparency.
When I began working in the biopharmaceutical industry more than 30 years ago, I understood the mandate, as a scientist, to use every tool we had to make a meaningful difference in improving peoples lives, to pursue breakthroughs, even cures, and eliminate serious diseases. HCV is one of those diseases.
Unfortunately, the U.S. healthcare system is not structured to easily absorb the up-front cost of a one-time cure, even if it results in significant savings over a patients lifetime. Further, our countrys complex drug supply chain means that a drugs list price does not always fully reflect the price paid by insurers let alone a patients out-of-pocket cost.
We are committed to working with key participants in the healthcare system, including insurers, policymakers and government officials, to lower the list prices of Harvoni and Epclusa moving forward.
-John Milligan
AREAS OF INTEREST
Don’t Miss: What Is The Most Common Genotype Of Hepatitis C
Gilead: Greed And Human Suffering
It is interesting when one Googles GILEAD the first thing we come up against the fictitious Fascist state in The Handmaidens Tale. So to find the roots of the name we need to look deeper, to the Hebrew origins of the name. We find in the original Hebrew text of the Bible, in the Book of Hosea that:
Gilead is a city of those who work iniquity it is stained with blood
Now if you modernize that description by replacing city with company we have to wonder about the choice of name for we come up with:
Gilead is a Company of those who work iniquity it is stained with blood
Which brings me to the point of this post, an email I received a while back from a guy in the USA who wanted GILEAD to know exactly how he felt about them and their work by writing Gileads Board of Directors a letter.
I have reproduced this email, and letter, below, in full. Its pretty intense but remember that it is written with the intensity of a person who is dying whilst knowing that the cure for his disease is on the shelf of the pharmacy a short distance away.
Fda Says Gilead Sciences’ Hepatitis C Drug Cures More Patients Faster With Few Side Effects
Matthew Perrone, The Associated PressOctober 23, 2013
WASHINGTON The Food and Drug Administration issued a positive review Wednesday for a highly anticipated hepatitis C drug from Gilead Sciences, saying the pill cures more patients in less time than currently available treatments.
The agency posted its review of Gileads sofosbuvir online ahead of a meeting Friday where government experts will vote on whether to recommend the drugs approval.
Between 3 million and 4 million people in the U.S. have hepatitis C, a blood-borne disease that causes liver damage and is blamed for 15,000 deaths a year. The drugs currently used to treat the virus cure about three-quarters of people and can take up to a year of treatment.
FDA said adding Gileads sofosbuvir to the standard drug cocktail cured 90 per cent of patients with the most common form of the virus in just 12 weeks.
The agencys reviewers state that the shorter 12-week duration translates into a better tolerated side effect profile, adding that no major safety issues associated with sofosbuvir have been identified to date.
Foster City, Calif.-based Gilead Sciences is one of a half-dozen companies working to develop more effective treatments for hepatitis C, and many analysts predict the companys drug will eventually outperform its competitors. The FDA is expected to make a decision on the drug by Dec. 8.
Gileads once-a-day pill appears to push the cure rate even higher.
Also Check: Can Hepatitis A Be Cured
The Medicine Nobel For Hep C Should Force Us To Think About Patents Patients Profits
The intertwined stories of HCV infections and sofosbuvir illustrate the big difference between innovating a drug to treat patients living with a disease and making sure all patients around the world have equal and affordable access to the drug.
Thomas Perlmann, Secretary of the Nobel Assembly at Karolinska Institutet and of the Nobel Committee for Physiology or Medicine, announces Harvey J? Alter, Michael Houghton and Charles M? Rice as the winners of the 2020 Nobel Prize in Physiology or Medicine during a news conference at the Karolinska Institute in Stockholm, Sweden, October 5, 2020. Photo: Claudio Bresciani/TT News Agency/via Reuters
Bengaluru: On October 5, the Nobel Prize for medicine was awarded to three molecular biologists who discovered the Hepatitis C virus in the late 1980s, and subsequently paved the way to reduce the risk of contracting it by helping develop other safeguards and treatments. Some 100-150 million people around the world currently suffer from Hepatitis C the WHO website says that 71 million people have chronic HCV infections. According to the 2017 Global Burden of Disease study, India had 4.83 lakh new HCV cases in 2014.
Also read: A Good Registry Means Accountable Clinical Trials. But Does India Have One?
Watch: Katherine Eban Interview: How Drug Makers Are Compromising the Lives of People
Also read: The Debate Around Drug Regulation Should Prioritise Price and Quality Equally
Fda Approves A Gilead Pill That Is First To Treat All Forms Of Hepatitis C
ATF maintained Gilead shifted the profits overseas to avoid taxes and pointed to a few signs to support its analysis. One, in particular, involved transferring economic rights to a key US patent for Sovaldi to an Irish subsidiary. This would have allowed Gilead to create a licensing arrangement that enabled the drug maker to report lower US profits and pay fewer taxes.
Three years ago, in fact, a Gilead executive told analysts the patent had been domiciled in Ireland, which would allow the US corporate tax rate to decline over time. As ATF noted, Gileads worldwide effective tax rate has since plummeted by 40 percent falling to 16.4 percent last year from 27.3 percent in 2013.
Gilead excels at tax dodging and price gouging, said congressman Lloyd Doggett, a Texas Democrat, in a statement. While a chief exporter of drug patents and profits to Ireland, it refuses to charge Americans the much lower drug prices of Ireland. Instead of innovation, it has spent more on stock buybacks for executives and shareholders than on research and development. Gileads formula for success: prices up, profits up, tax avoidance up. We dont need research to know this about drug effectiveness: an unaffordable drug is always 100 percent ineffective.
A Gilead spokeswoman declined to comment.
For this reason, one tax expert believes that while the ATF report may correctly highlight Gileads tax gambits, the company is not really the issue at hand.
Recommended Reading: What Happens When You Get Hepatitis C
Gilead: Stop Blocking Access To Hepatitis C Treatmentthousands Of Activists Urge Company To Change Its Policies On Eve Of Shareholder Meeting
New York, May 5, 2015 On the eve of pharmaceutical company Gilead Sciences annual shareholder meeting, thousands of people who have hepatitis C virus and HCV/HIV coinfection, with their allies and physicians, have demanded that Gilead change its policies that deny access to treatment for millions of people in developing countries.
Gilead must immediately stop blocking access to affordable generic hepatitis C treatmentand abolish egregious anti-diversion measures that violate patient rights, said Othman Mellouk of the International Treatment Preparedness Coalition.
Hepatitis C is a global public health crisiscalled a viral time bomb by the World Health Organization with at least 150 million people living with the disease. Untreated hepatitis C can progress to cirrhosis, liver failure, and liver cancer. Every year, at least 700,000 people die from these complicationsalthough HCV can be easily cured with just 12 weeks of oral direct-acting antiviral drugs. But most people dont have access to the DAAs sofosbuvir and the sofosbuvir/ledipasvir combination because Gilead prices them exorbitantly, and many developing countries are excluded from Gileads treatment expansion plans.
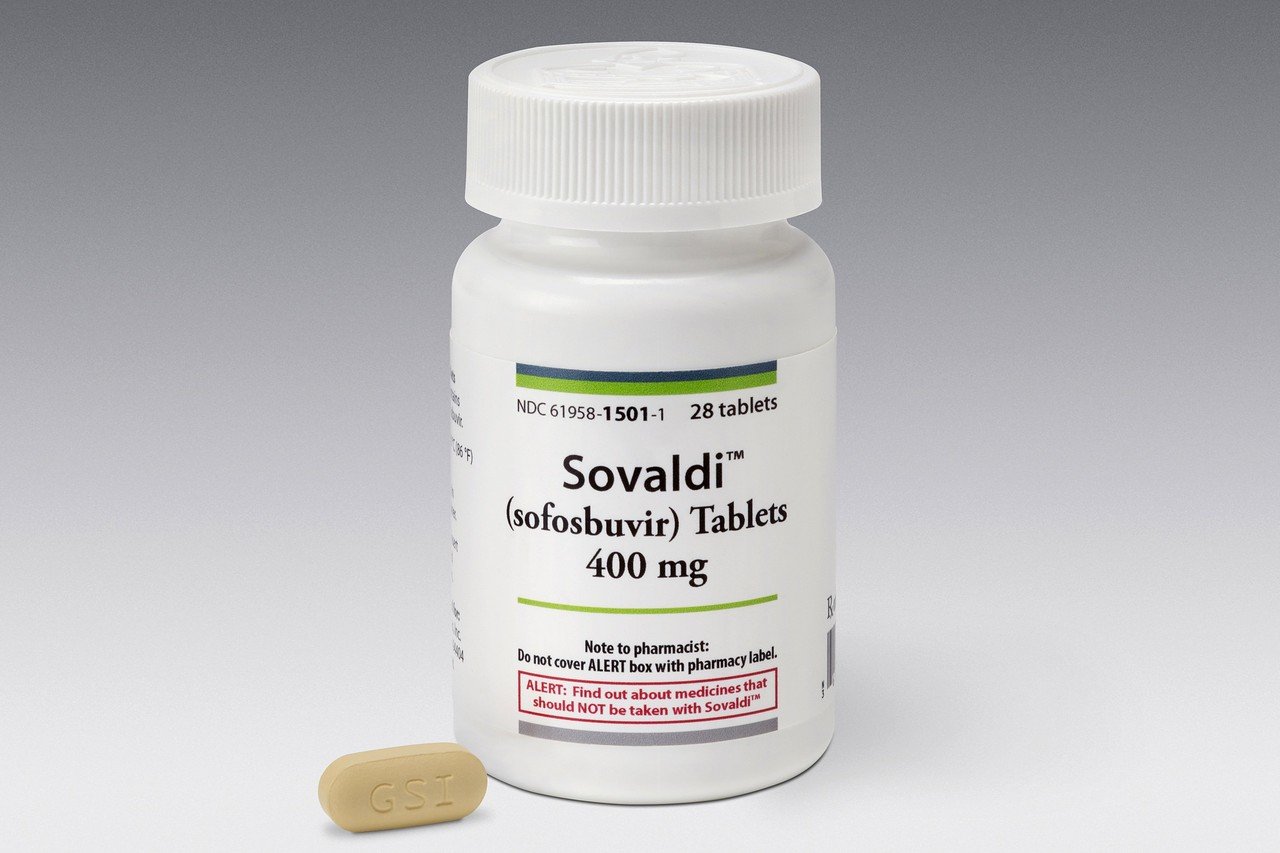
Sofosbuvir, priced in the United States at $84,000 per 12-week treatment course, is unaffordable even for high-income countries. Meanwhile, research from Liverpool University shows that the 12-week course can be mass-produced for just US$101.
A petition presented to Gilead today stated:
We Believe In Equitable Access To Lifesaving Hcv Treatment For All Who Need It
CONTACTS:
# # #
NOTE TO EDITORS:
Link to petition: http://www.petitionbuzz.com/PETITIONS/HEPC
For more information on the minimum cost of HCV DAA production: van de Ven, N., Fortunak, J., Simmons, B., Ford, N., Cooke, G. S., Khoo, S. and Hill, A. , Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology, 61: 11741182. doi: 10.1002/hep.27641
For more information on Gileads voluntary license program: http://www.msfaccess.org/content/fact-sheet-gileads-chronic-hepatitis-c-treatment-restrictions
For more information on Gileads anti-diversion program:http://www.msfaccess.org/content/barriers-access-and-scale-hepatitis-c-hcv-treatment-gileads-anti-diversion-program
Also Check: How Can Hepatitis C Be Transmitted Sexually
Foster City Firm May Break Record For New Drug Sales
Gilead Sciences’ breakthrough hepatitis C drug may have smashed the record books.
The Foster City company said Sovaldi generated nearly $2.3 billion in the first three months of the year, easily surpassing analysts’ estimates of $1 billion. That would make Sovaldi the best-selling new drug of all time, according to some analysts.
“This is the biggest launch of a new drug in first-quarter sales that we’re aware of,” said Michael Yee, an analyst for RBC Capital Markets. “I think it’s a testament to the transformation breakthrough this drug is providing, which is essentially a cure in a pill.”
The figures released Tuesday were a clear triumph for Gilead, which spent more than $11 billion to purchase and develop the technology that produced Sovaldi. The drug treats a virus that inflicts 130 million people worldwide, including 3 million Americans.
Sovaldi generated half of Gilead’s roughly $5 billion overall sales in the first quarter. Those revenues are more than double the amount from the same period last year. So far, 30,000 patients have started to use Sovaldi, with many more to follow, company officials said.
Anticipating strong sales, investors sent Gilead’s shares up 1.8 percent to nearly $73 a share Tuesday.
“I couldn’t be more proud of the teams at Gilead who rapidly brought this product to market,” Gilead CEO John Martin told investors in a conference call.
A Letter To Gilead Sciences
The release of Gileads Hepatitis C medication was the most profitable event in the history of the pharmaceutical industry. Whilst there is nothing wrong with making a healthy profit the problems arise when obscene profits are made on the back on terrible human suffering through the manipulation of markets and marketing laws.
You May Like: How To Treat Alcoholic Hepatitis
Breakthrough Hepatitis C Drug From Gilead Sciences Offers Faster Cure And Fewer Side Effects
Matthew Perrone, The Associated PressDecember 6, 2013
WASHINGTON Federal health officials have approved a highly anticipated hepatitis C drug from Gilead Sciences Inc. that is expected to offer a faster, more palatable cure to millions of people infected with the liver-destroying virus.
The Food and Drug Administration said Friday it approved the pill Sovaldi in combination with older drugs to treat the main forms of hepatitis C that affect U.S. patients.
Current treatments for hepatitis C can take up to a year of therapy and involve weekly injections of a drug that causes flu-like side effects. That approach only cures about three out of four patients. Sovaldi is a daily pill that in clinical trials cured roughly 90 per cent of patients in just 12 weeks, when combined with the older drug cocktail.
Between 3 million and 4 million Americans are estimated to carry the blood-borne virus, though most do not even know they are infected. Others have tested positive but are waiting for more effective treatments to become available. Hepatitis C symptoms may not appear until two or three decades after infection, though the virus can cause liver failure, cirrhosis and cancer if left untreated.
Dr. Donald Jensen of the University of Chicago said hes optimistic that new drugs like Sovaldi will increase treatment of the disease, which is blamed for 15,000 U.S. deaths per year.
Gileads once-a-day pill pushes the cure rate much higher.
Maker Of $1000 Hepatitis C Pill Was Focused On Profits Not Patients Report Finds
WASHINGTON The makers of a breakthrough hepatitis drug put profits before patients in pricing the $1,000 pill thats become a symbol of the excessive cost of medications, Senate investigators said Tuesday.
A bipartisan report from the Senate Finance Committee concluded that California-based Gilead Sciences Inc. was focused on maximizing revenue for its hepatitis C medications, even as the companys own analysis showed a lower price would allow more patients to be treated.
The lawmakers who led the investigation said its a warning about what could happen with other innovative treatments for cancer, diabetes, Alzheimers and HIV.
Im telling you, this is the future, said Sen. Ron Wyden, D-Ore.
When we see the market reaction especially the limitations on access, we have to be concerned, said Sen. Chuck Grassley, R-Iowa.
In a statement, Gilead said it disagreed with the reports conclusions. Gilead stock was down slightly most of the day Tuesday, as broader financial markets posted solid gains.
The companys first breakthrough drug, Sovaldi, was priced at $1,000 per pill, or $84,000 for a course of treatment. Gilead has since introduced a more expensive, next-generation pill called Harvoni, highly effective and simpler for patients to use. Its priced at $94,500 for a course of treatment.
Other findings and conclusions from the report:
Recommended Reading: Causes Of Hepatitis B And C
Gilead’s Asegua Therapeutics Selected To Partner With Health Department For Hepatitis C Drug Subscription Model
The Louisiana Department of Health and Department of Corrections announced today their selection of Asegua Therapeutics LLC as their hepatitis C subscription model pharmaceutical partner, to provide the State with unrestricted access to its direct-acting antiviral medication.
The Departments will now establish a contract with Asegua Therapeutics to make its medication, the authorized generic of Epclusa® , available to hepatitis C patients enrolled in Medicaid and to treat incarcerated patients over the next five years.
With this partnership, the Departments of Health and Corrections will have five years of unrestricted access to Asegua Therapeutics highly effective, direct-acting antiviral treatment. The medication has an overall cure rate of 98 percent across all six main types of hepatitis C.
Dr. Rebekah Gee, secretary of the Louisiana Department of Health, said with todays announcement Louisiana has reached a significant milestone toward establishing the nations first subscription model for hepatitis C treatment.
The next step is to complete the contract between Asegua and our agency, said Gee. We were extremely pleased that three manufacturers offered proposals, with the plan submitted by Asegua offering us a clear path forward to offer a hepatitis C cure to our most vulnerable patients.
James LeBlanc, secretary of the Louisiana Department of Public Safety and Corrections, said the partnership will help patients who are incarcerated be cured of hepatitis C.
A Growing Group Of Doctors Are Big
“Because we have a cure, the costs are paid in a short period of time,” he says. “Our payment systems have a real tough time with that because they’re used to these sorts of long-term, chronic therapies that pay a little bit at a time but, over time, cost a lot more.” In other words, it can cost a lot more to treat diabetes or multiple sclerosis over a lifetime than to cure hepatitis C in three months.
From a public health perspective, buying Gilead isn’t a bad idea, says Sara Fisher Ellison, an economics lecturer at MIT.
But that assumption rests on the notion that the government would buy the company’s shares at full price on the open market. That would avert any reaction from drugmakers or other companies who would fear that the government could use its power just to force them to lower prices.
“The reason this proposal could work is that right now the drugs are only serving a fraction of the potential patients,” Fisher Ellison says, “and there’s a big public health benefit to serving all of the patents. That’s something that Gilead doesn’t take into account in its pricing policy, but it’s something that a government does take into account.”
The idea is a long shot of course.
But Higginbotham, who is still waiting in Colorado for his chance to take the hepatitis C drug, sees an opening with Donald Trump in the Oval Office.
“With our present administration,” he says, “a hostile takeover might be just up his alley.”
You May Like: Signs You Have Hepatitis B