Ed Screening Program Design
All ED patients 18 years and born after 1945, who were having blood drawn for any clinical purpose and who did not have a positive HCV RNA test result in the EHR, underwent opt-out HCV screening . Upon entering any laboratory order into the EHR, a BPA alerted the ED provider that the patient was eligible for HCV screening. This BPA functioned as both an alert and a hard stop for which providers were required to respond to continue with the order entry. The ED provider could, on behalf of the patient, accept, or defer testing. If deferred by the nurse, the BPA would appear again on subsequent phlebotomy orders and if deferred by the physician, it would not appear for the duration of the current encounter but would reappear on subsequent ED visits. On the other hand, if accepted, the BPA generated HCV screening discharge documentation documenting patient verbal authorization for testing, and triggered an order in the EHR for HCV testing and printed labels for specimen collection.
Summary Of The Literature
For the all-adult review, the initial literature search yielded 4,867 studies. Twenty-nine duplicates were identified. Of 4,838 unique studies, 4,170 were deemed irrelevant by title/abstract screening, resulting in 668 full texts for review. Among these, 368 studies had data available to extract.
For the pregnancy review, the initial literature search yielded 1,500 studies. Two duplicates were identified. Of 1,498 unique studies, 1,412 were deemed irrelevant by title/abstract screening, resulting in 86 full texts for review.
The supplementary review yielded an additional 1,038 and 195 studies among all adults and pregnant women, respectively. Of these, 912 and 168 , respectively, were deemed irrelevant by title/abstract screening, resulting in 126 and 27 , respectively, full texts for review. One study was added to the pregnant women review outside of the formal literature search .
Considering all 104 applicable studies, the median anti-HCV positivity prevalence among all adults was 6.6% . Median anti-HCV positivity prevalence was 1.7% for the general population , 7.5% for ED patients , 3.3% for birth cohort members , 9.3% for others/multiple risk factors , 54.2% for persons who use drugs , 5.2% for persons with HIV or sexual risk , and 4.7% for immigrants . Considering 26 applicable studies among pregnant women, median anti-HCV positivity prevalence was 1.2% .
Effective Treatments Are Available For Hepatitis C
New medication to treat for HCV have been approved in recent years. These treatments are much better than the previously available treatment because they have few side effects and do not need to be injected. There are several direct-acting antiviral HCV treatments that cure more than 95% of people who take them in 8 to 12 weeks. HCV treatment dramatically reduces deaths among people with HCV infection, and people who are cured of HCV are much less likely to develop cirrhosis or liver cancer.
Take Action! CDCs National Prevention Information Network Service Locator helps consumers locate hepatitis B and hepatitis C prevention, care, and treatment services.
Recommended Reading: Hepatitis C What Does It Do
What Is Hepatitis C
Hepatitis C is a liver disease that results from an HCV infection. The virus passes on through contact with blood from someone who has the infection.
Hepatitis C can be acute or chronic. Chronic hepatitis C can lead to more serious complications, such as cirrhosis and liver cancer.
After contracting the infection, nearly 80% of people have no symptoms. Any symptoms typically take
Aasld/idsa Hcv Testing Guidance
The American Association for the Study of Liver Diseases and Infectious Diseases Society of America guidance for hepatitis C addresses HCV testing in the section HCV Testing and Linkage to Care. The AASLD/IDSA recommends one-time, routine, opt out HCV testing for all individuals aged 18 years and older, one-time testing for persons younger than age 18 who have increased risk for acquiring HCV, periodic testing for persons who have risk activity for acquiring HCV, and annual testing for men with HIV who have condomless sex with men men who have sex with men and are on HIV preexposure prophylaxis and people who inject drugs . The AASLD/IDSA recommendations for testing incorporate birth-cohort screening as well as testing based on risk behaviors, risk exposures, and medical conditions associated with acquisition of HCV.
Read Also: Hepatitis B Is Much More Easily Transmitted Than Hiv
Universal Screening For Hepatitis C Virus In The Ed Using A Best Practice Advisory
| UC Davis Health, Department of Emergency Medicine, Sacramento, California | |
| Tasleem Chechi, MPH | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
| Kavian Toosi, MD | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
| Bilawal Mahmood, | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
| Dillon Meehleis, | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
| Michella Otmar, NP, MPH | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
| Nam Tran, PhD | UC Davis Health, Department of Pathology and Laboratory Medicine, Sacramento, California |
| Larissa May, MD, MSPH, MSHS | UC Davis Health, Department of Emergency Medicine, Sacramento, California |
Hiv And Hepatitis C Coinfection
HCV infection is common among people with HIV who also inject drugs. Nearly 75% of people living with HIV who report a history of injection drug use are co-infected with HCV. All people who are diagnosed with HIV are recommended to be tested for HCV at least once. People living with HIV are at greater risk for complications and death from HCV infection. Fortunately, direct acting antivirals that are used to treat HCV work equally well in people with and without HIV infection. For more information about HIV and HCV coinfection, visit the HIV.govs pages about hepatitis C and HIV coinfection.
You May Like: Symptoms Of Hepatitis B In Males
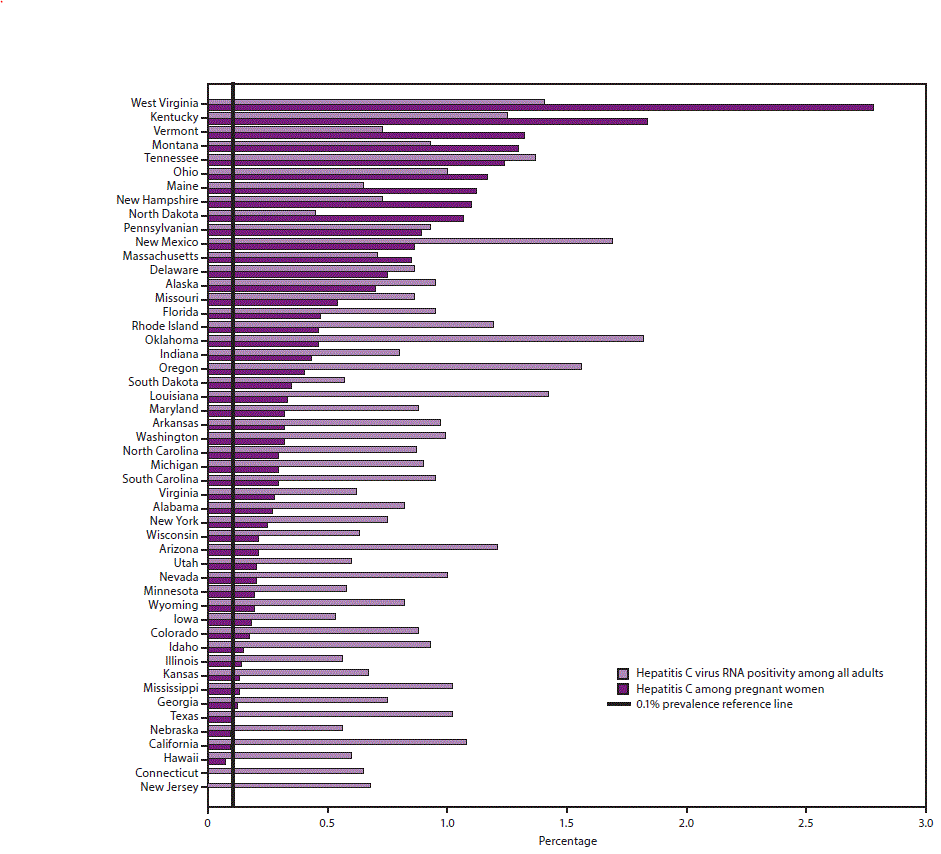
Cdc Recommendations For Hcv Screening
On April 10, 2020, the Centers for Disease Control and Prevention issued new recommendations for hepatitis C screening among adults in the United States . This new guidance augments prior CDC guidance on HCV screening with two new major recommendations: all adults aged 18 years and older should have HCV screening at least once in their lifetime, except in settings where the prevalence of HCV infection is less than 0.1%, and HCV screening should be performed for all pregnant persons during each pregnancy, except in settings where the prevalence of HCV infection is less than 0.1%. The CDC continues to recommend screening persons for HCV regardless of age if risk factors for acquiring HCV are present, with repeat periodic screening in persons who have ongoing risk for acquiring HCV. These new CDC HCV screening recommendations expand prior guidance that recommended routine HCV screening for all persons born between 1945-1965.
Population Health Research Capsule
What do we already know about this issue?
Hepatitis C virus screening guidelines recommend screening adults aged 1879 years. HCV testing has been explored in the ED but remains controversial.
What was the research question?
What is the utility of a universal best practice alert-based ED HCV screening program?
What was the major finding of the study?
A universal best practice alert-based ED HCV screening program drastically increased HCV testing and diagnosis.
How does this improve population health?
The ED has a high-risk population with HCV prevalence well above the national average. ED screening programs could improve diagnosis and linkage to treatment.
You May Like: How Do Catch Hepatitis C
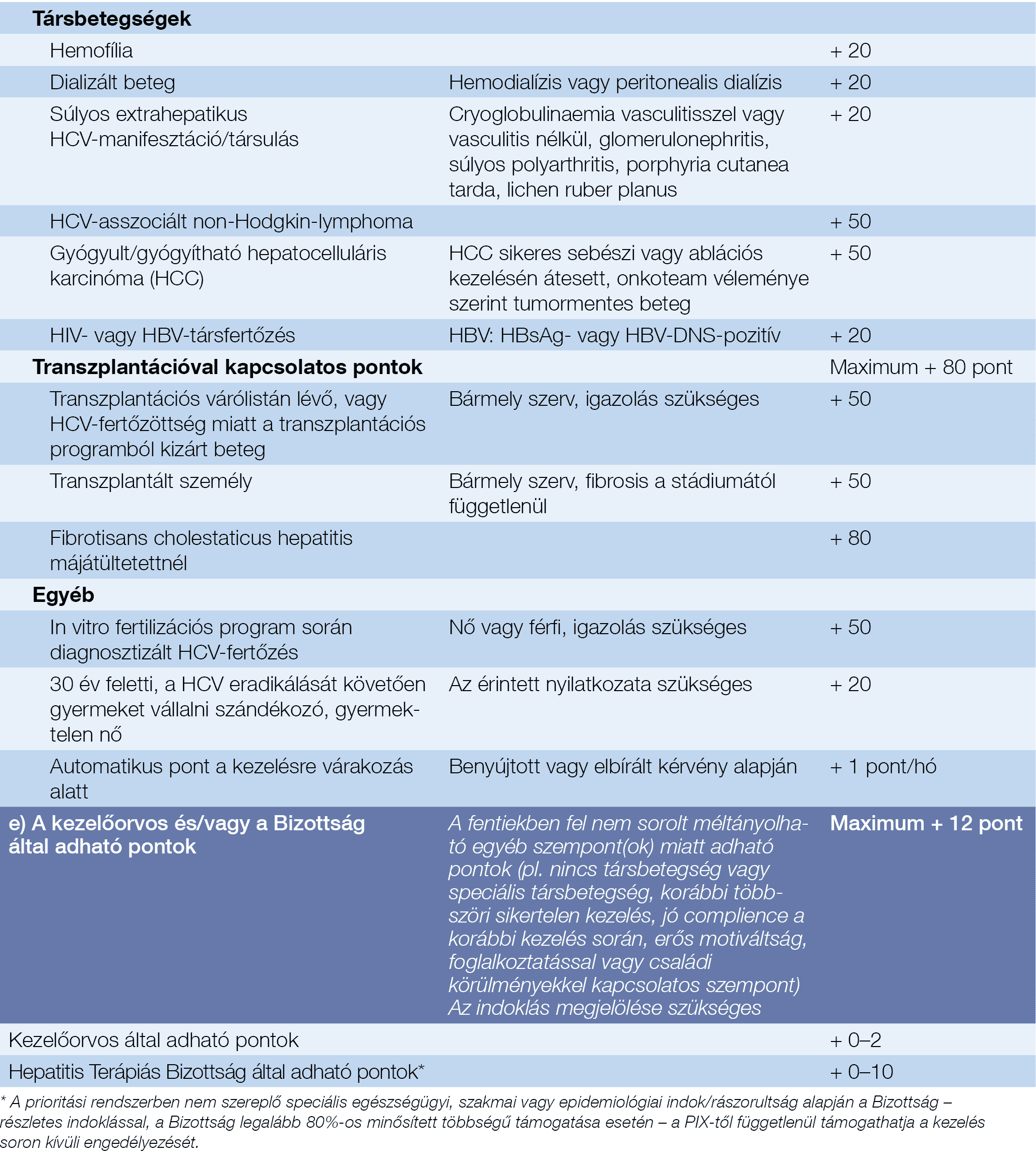
Recommendations For Testing Managing And Treating Hepatitis C Virus Infection
The advent of safe, well-tolerated, and highly efficacious direct-acting antiviral therapy for hepatitis C virus infection has ushered in an era in which elimination of hepatitis C is conceivable. In 2016, the World Health Organization proposed a global health sector strategy to eliminate hepatitis C as a public health threat by 2030 and developed an action plan to facilitate this goal. In response to the WHO action plan, the National Academies of Science, Engineering, and Medicine developed a US strategy for the elimination of hepatitis C. Key elements of the elimination plan include improved detection of undiagnosed cases, increased linkage and access to care for newly diagnosed persons, and expanded treatment access. Many of the recommendations included in the latest update to the HCV guidance and highlighted herein align with and support the goals of the NASEM and WHO strategies to move from control to eventual elimination of hepatitis C. Topics addressed include universal and risk-based hepatitis C screening, simplified treatment algorithms for treatment-naive adults without cirrhosis or with compensated cirrhosis, hepatitis C management in the pediatric population, acute hepatitis C testing and management, and transplantation of organs from HCV-viremic donors into HCV negative recipients.
Current Hepatitis C Testing Recommendations
Recently, several organizations have issued hepatitis C virus screening recommendations. In general, major guidelines now recommend routine one-time universal HCV testing for adults 18 years of age and older, routine HCV screening of pregnant individuals, screening younger persons at risk of acquiring HCV, and repeat screening for those with ongoing risk for HCV acquisition.
You May Like: How Is Hepatitis C Contracted
Who Else Should Get Tested
Anyone who has hepatitis C symptoms should speak with a healthcare professional.
The HCV transmits via contact with blood, and it can pass from person to person through:
- sexual intercourse with someone who has the infection
- sharing personal items that may have come into contact with blood, such as razors
- tattooing in conditions that do not meet health and safety standards
- needle prick accidents in healthcare settings
Anyone who may have come into contact with the HCV should speak with a healthcare professional about having a test.
Persons At Risk For Hcv Infection
IDU is the most common means of HCV transmission in the United States. Invasive medical procedures pose risks for HCV infection when standard infection-control practices are not followed . Health carerelated hepatitis C outbreaks also stem from drug diversion . Although HCV infection is primarily associated with IDU, high-risk behaviors , primarily among persons with HIV, are also important risk factors for transmission . Other possible exposures include sharing personal items contaminated with blood , unregulated tattooing, needlestick injuries among health care personnel, and birth to a mother with hepatitis C. Receipt of donated blood, blood products, and organs was once a common means of transmission but is now rare in the United States .
Read Also: Screening For Hepatitis C Icd 10
Determining The Prevalence Threshold For The Recommendations
Although the intent of public health screening is usually to identify undiagnosed disease, many persons previously diagnosed with hepatitis C are not appropriately linked to care and are not cured of their HCV infection, thereby representing an ongoing source of transmission. Therefore, the prevalence threshold of 0.1% should be determined on the basis of estimates of chronic hepatitis C prevalence, regardless of whether hepatitis C has been diagnosed previously.
How Is Hepatitis C Transmitted
Because HCV is primarily spread through contact with infected blood, people who inject drugs are at increased risk for HCV infection. HCV can also be transmitted from an infected mother to child at the time of birth, from unregulated tattoos or body piercings, and from sharing personal items that may be contaminated with infected blood, even in amounts too small to see. Much less often, HCV transmission occurs through sexual contact with an HCV-infected partner, especially among people with multiple sex partners and men who have sex with men. Currently in the United States, health care related transmission of HCV is rare, but people can become infected from accidental needle sticks and from breaches in infection control practices in health care facilities.
Also Check: Hepatitis B Or C Symptoms
Assessment Of Magnitude Of Net Benefit
The USPSTF concludes with moderate certainty that screening for HCV infection in adults aged 18 to 79 years has substantial net benefit.
See Table 1 and Table 2 for more information on the USPSTF recommendation rationale and assessment. For more details on the methods the USPSTF uses to determine net benefit, see the USPSTF Procedure Manual.8
Characteristics Of Study Subjects
Patient characteristics stratified by study period are summarized in Table 1. The median age of patients was similar between periods . Patients screened were more likely to be male in the pre-BPA period . The proportions of patients within each racial or ethnic category were similar between study periods.
Table 1Patient characteristics by study period.
| Characteristic | |
|---|---|
| 3,351 | 0.05 |
Age reported as median and analyzed between study periods using Mann-Whitney U test. Categorical variables reported as number and analyzed between study periods using Fishers exact test.1Gender data missing for two patients in post-BPA period.2Race data missing for 6 patients in pre-BPA and 200 patients in post-BPA group. Ethnicity data were missing in 16 patients in the pre-BPA group and 177 patients in the post-BPA group.BPA, best practice advisory.
You May Like: What Does Hepatitis C Do To Your Body
Clinical Features And Natural History
Persons with acute HCV infection are typically either asymptomatic or have a mild clinical illness like that of other types of viral hepatitis . Jaundice might occur in 20%30% of persons, and nonspecific symptoms might be present in 10%20% of persons. Fulminant hepatic failure following acute hepatitis C is rare. The average time from exposure to symptom onset is 212 weeks . HCV antibodies can be detected 410 weeks after infection and are present in approximately 97% of persons by 6 months after exposure. HCV RNA can be detected as early as 12 weeks after exposure. The presence of HCV RNA indicates current infection .
Diagnosis And Hepatitis C Elimination
In one report, the National Academies of Sciences, Engineering, and Medicine explored the feasibility of hepatitis C elimination and concluded that hepatitis C could be eliminated as a public health problem in the United States, but that substantial obstacles exist . In another report, specific actions were recommended to achieve elimination considering information, interventions, service delivery, financing, and research . These reports were the culmination of decades of progress in the development of HCV infection diagnostic and therapeutic tools.
The 2012 CDC guidelines recommended that pregnant women be tested for hepatitis C only if they have known risk factors . However, in 2018, universal hepatitis C screening during pregnancy was recommended by the American Association for the Study of Liver Diseases and IDSA . This report expands hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is < 0.1%.
Don’t Miss: Diet Plan For Hepatitis Patient
What Are Cdcs Hepatitis C Screening Recommendations
All patients 18 years and older should be screened for hepatitis C at least once in their lifetime, except in settings where the prevalence of HCV infection is < 0.1%.
Patients with recognized exposures should be tested for hepatitis C regardless of age or setting prevalence, and regular periodic testing should continue as long as risk persists.
How Many People Have Hepatitis C
During 2013-2016 it was estimated that about two and half million people were chronically infected with HCV in the United States. The actual number may be as low as 2.0 million or as high as 2.8 million.Globally, hepatitis C is a common blood-borne infection with an estimated 71 million people chronically infected according to the World Health Organization .
You May Like: What Are The Signs Of Hepatitis B
All Adults Ages 18 To 79 Should Be Screened For Hepatitis C New Recommendation Says
The US Preventive Services Task Force now recommends screening for hepatitis C infection in all adults ages 18 to 79 without known liver disease, regardless of their risk.
This updated recommendation, on Monday, expands the task forces previous 2013 recommendation, which was to screen only adults born between 1945 and 1965 and others at high risk for infection.
People with hepatitis C do not always feel sick and may not know they have it, Task Force chair Dr. Douglas K. Owens, an author of the recommendation and a general internist, said in a news release on Monday.
Screening is key to finding this infection early, when its easier to treat and cure, helping reduce illnesses and deaths, said Owens, who is also an investigator at the Center for Innovation to Implementation at the Veterans Affairs Palo Alto Health Care System.
The USPSTF commissioned a systematic review of current research and evidence on the hepatitis C virus, or HCV, in order to update its prior recommendation. Based on that review, the task force found that screening more adults and teens has substantial net benefit since detecting infection early can lead to the early use of effective treatments and interventions.
It is time to revisit the effective but now outdated baby boomer screening recommendations, and the updated recommendations from the USPSTF are welcome, they wrote.
Virus Description And Transmission
HCV is a small, single-stranded, enveloped RNA virus in the flavivirus family with a high degree of genetic heterogeneity. Seven distinct HCV genotypes have been identified. Genotype 1 is the most prevalent genotype in the United States and worldwide, accounting for approximately 75% and 46% of cases, respectively . Geographic differences in global genotype distribution are important because some treatment options are genotype specific . High rates of mutation in the HCV RNA genome are believed to play a role in the pathogens ability to evade the immune system . Prior infection with HCV does not protect against subsequent infection with the same or different genotypes.
Also Check: Who Should Get Hepatitis B Vaccine
Cdc Recommendations For Hepatitis C Screening Among Adults In The United States
- Universal hepatitis C screening:
- Hepatitis C screening at least once in a lifetime for all adults aged 18 years and older, except in settings where the prevalence of HCV infection is less than 0.1%*
- Hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is less than 0.1%*
- Any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many persons may be reluctant to disclose stigmatizing risks