When Should I Get Hepatitis C Testing
When used for early detection in patients without symptoms of hepatitis C, screening is recommended at least once for all adults aged 18 years or older, except in locations with very low prevalence of HCV. Screening is also recommended during pregnancy and for patients of any age with risk factors for HCV infection. In patients with risk factors, periodic screening is recommended for as long as risk factors persist.
Risk factors for HCV include:
- Current or past injectable drug use
- Having a blood transfusion or organ transplant before July 1992
- Receiving kidney dialysis
- Pain in the abdomen or joints
- Nausea, vomiting, or loss of appetite
- Jaundice or yellowish skin and eyes
Hepatitis C testing may also be performed when liver tests are abnormal or when diagnosing the cause of existing liver damage.
How Is A Person Tested For Hepatitis C
A viral-load test is used to check for hepatitis C in the bloodstream. Usually, hepatitis C virus can be found in a persons bloodstream two weeks after he or she becomes infected.
*Except in case of recent risk or in people with a weakened immune system**During the first six months after HCV infection, a person may spontaneously clear the virus if there was a recent risk, repeat viral-load testing to confirm chronic hepatitis C infection
Concurrent Administration Of Vaccines
HB-containing vaccines may be administered concomitantly with other vaccines or with HBIg. Different injection sites and separate needles and syringes must be used for concurrent parenteral injections.
Refer to Timing of Vaccine Administration in Part 1 for additional information about concurrent administration of vaccines.
Read Also: What Are The Side Effects Of Hepatitis B
Preparation Prior To Transport
Label the specimen container with the patients full name, date of collection and one other unique identifier such as the patients date of birth or Health Card Number. Failure to provide this information may result in rejection or testing delay.
Centrifuge if using SST. Place specimen in biohazard bag and seal. Specimens should be stored at 2-8°C following collection.
Specimens more than the following number of days post collection will not be tested:
- > 6 days for Hepatitis B surface antigen
- > 7 days for Hepatitis B e Antigen and Hepatitis B e Antibody
- > 10 days for Hepatitis B core Antigen and Hepatitis B surface Antibody
Discusses Physiology Pathophysiology And General Clinical Aspects As They Relate To A Laboratory Test
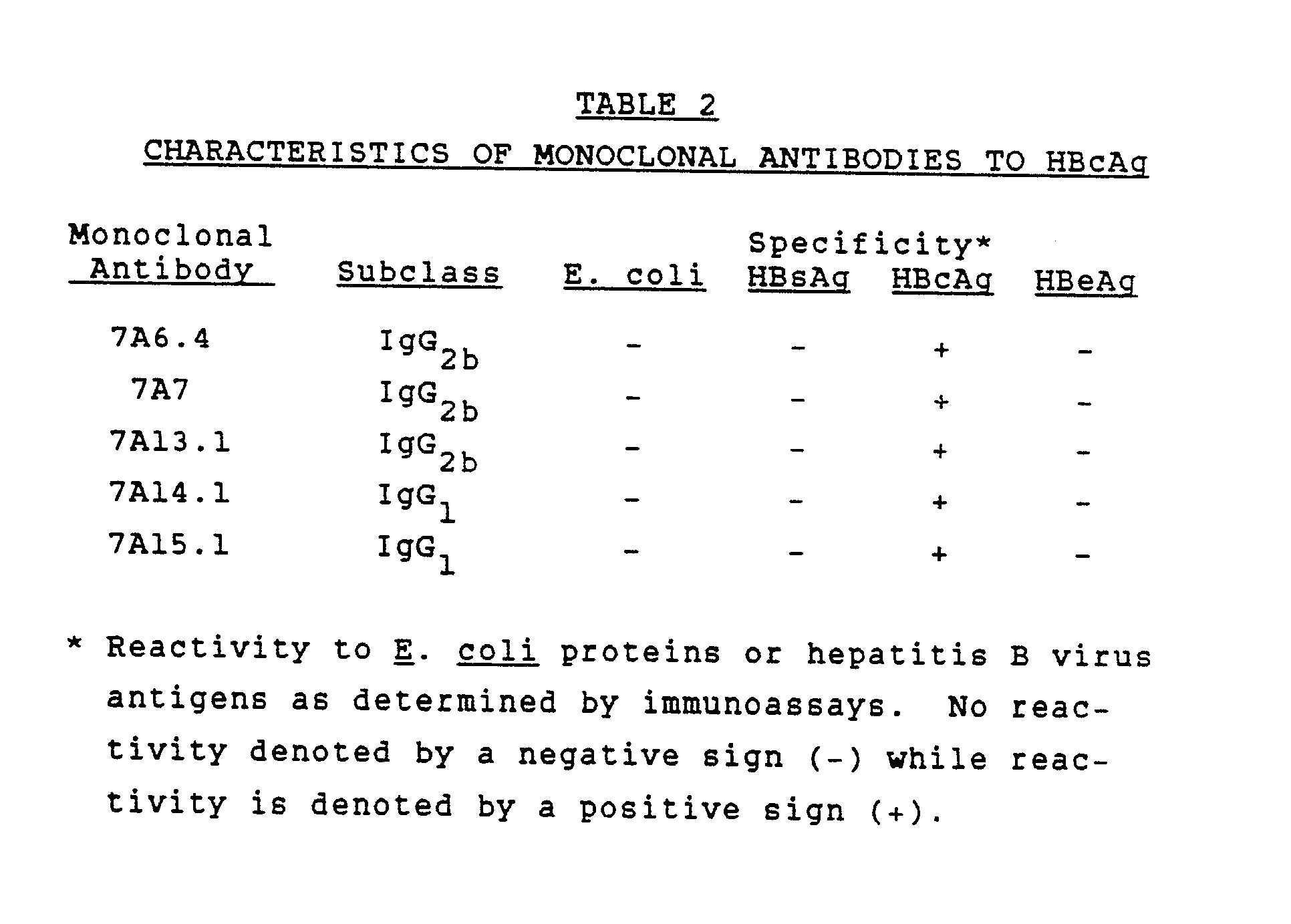
Hepatitis B core antibodies appear shortly after the onset of symptoms of hepatitis B infection and soon after the appearance of hepatitis B surface antigen . Initially, anti-HBc Ab consist almost entirely of the IgM class, followed by appearance of anti-HBc IgG, for which there is no commercial diagnostic assay.
The anti-HBc total antibodies test, which detects both IgM and IgG antibodies, and the test for anti-HBc IgM antibodies may be the only markers of a recent hepatitis B infection detectable in the “window period.” The window period begins with the clearance of HBsAg and ends with the appearance of antibodies to hepatitis B surface antigen . Anti-HBc total Ab may be the only serologic marker remaining years after exposure to hepatitis B.
This assay is FDA-approved for in vitro diagnostic use and not for screening cell, tissue, and blood donors.
You May Like: How Does One Catch Hepatitis B
How Much Does The Test Cost
The cost of hepatitis C testing depends on the tests that are performed, where the test is conducted, and a patients health insurance coverage. When testing is ordered by a doctor, patients with health insurance may find it helpful to discuss the cost of hepatitis C testing with their insurance company. In addition to the cost of testing, there may be other out-of-pocket costs such as copays and deductibles.
For patients without health insurance, or for whom insurance doesnt cover the cost of testing, it may be helpful to discuss the cost of hepatitis C testing with a doctor or hospital administrator.
At-home hepatitis C testing starts around $49. Some at-home kits test for multiple types of viral hepatitis at once, with the cost of these panels starting around $80.
What Do The Results Mean
There are two results from a hepatitis C antibody test.
- A non-reactive or negative test result means that the person does not have the virus. The exception is if someone has come into contact with the virus recently, such as through contaminated blood. If this is the case, they will need to have another test.
- A reactive or positive test result means that the person has had the virus at some point but does not mean that they still have it. Further tests will be needed to check whether the virus is still active in the body and if treatment will be required.
Once diagnosed with hepatitis C, a person will need to undergo a series of different tests to see how the virus has affected their body.
These tests will check for any liver damage, identify how well the liver is working, and help a healthcare professional to decide on treatment.
Hepatitis C is treated with medication known as an antiviral. It gets this name because it aims to clear the virus out of the body.
Another aim of the medication is to slow down damage to the liver. It may also reduce the chance of a person getting liver cancer or developing serious liver scarring, known as cirrhosis.
A person with hepatitis C will require regular testing during treatment to see how well the medication is working. Keeping healthy, getting enough sleep, and avoiding drugs and alcohol can help treatment to work.
Also Check: Hepatitis C Contagious Through Urine
Recommended Laboratory Evaluation Prior To Referral
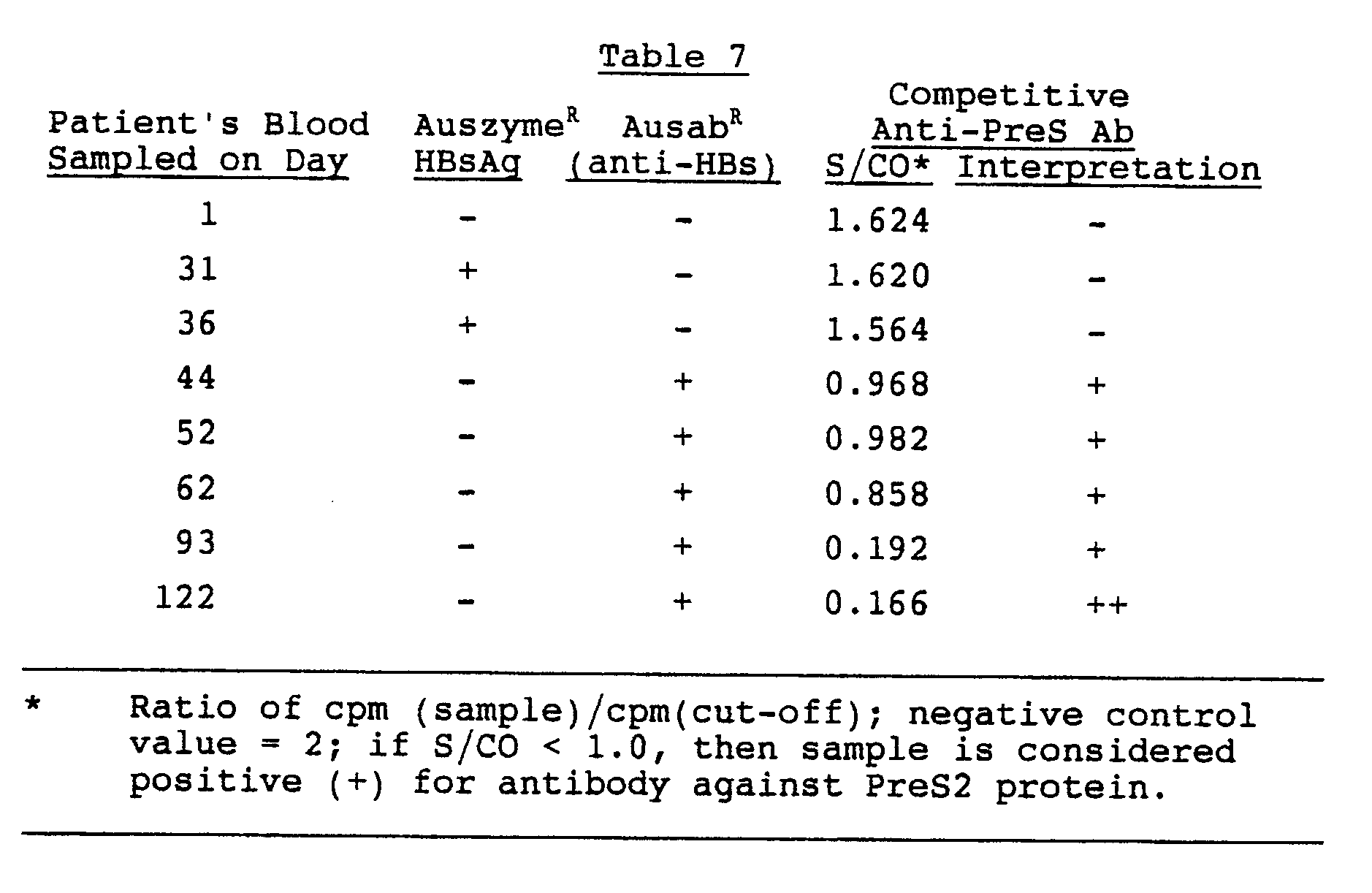
All persons referred for further evaluation and management of HCV infection should have a confirmed positive HCV RNA level, preferably a quantitative HCV RNA level and not a qualitative HCV RNA level. It is ideal, but not imperative, that the clinician who makes the diagnosis of HCV infection can perform some preliminary tests to provide advanced information in anticipation of the initial referral visit. These initial preliminary tests include an HCV genotype, tests of synthetic liver function , hepatic inflammation , and assays to detect relevant coinfection . For primary care providers taking on a more comprehensive role for the initial evaluation and management, see Module 2, Lesson 1 for a detailed discussion in the Core Concept Initial Evaluation of Persons with Chronic Hepatitis C.
Can I Take The Test At Home
At-home hepatitis C tests are available that allow patients to collect a blood sample at home and mail it to a laboratory for testing. Test samples are collected through pricking a finger with a sharp object, called a lancet, thats included in the test kit.
At-home HCV testing is a form of hepatitis C antibody testing and does not test for hepatitis C RNA or the strains genotype. Testing for hepatitis C at home is not a substitute for testing performed by a health care professional, and positive test results may need to be confirmed by laboratory-based testing.
Also Check: How Do You Contract Hepatitis C
Strategies For Improving Linkage To Care
Attempts at the public health level to implement an HCV testing and linkage-to-care program have shown that additional funds can be used to leverage existing program and provider networks. The CDC and other organizations are actively working to explore strategies, such as the Hepatitis Testing and Linkage to Care initiative, to enhance linkage to care for persons infected with HCV. It should also be noted that patients who have been previously diagnosed many years ago in the interferon era may have been counseled to not seek treatment given the relatively poor efficacy, long duration, and high rate of adverse effects associated with interferon-based therapy. These patients may require more intensive outreach efforts to educate and update on new greatly improved medications that are now available.
Guidance On Reporting Adverse Events Following Immunization
Vaccine providers are asked to report, through local public health officials, any serious or unexpected adverse event temporally related to vaccination. An unexpected AEFI is an event that is not listed in available product information but may be due to the immunization, or a change in the frequency of a known AEFI.
Refer to Reporting Adverse Events Following Immunization in Canada and Adverse events following immunization in Part 2 for additional information about AEFI reporting.
Recommended Reading: Is Hepatitis B And Hiv The Same
Correlation Between Hcv Core Antigen Level And Hcv Rna Level
Numerous studies have explored the correlation between HCV RNA values and HCV core antigens also in relation to genotypes, and overwhelmingly high correlations were reported between 0.7 to > 0.9 for r-values. This would indicate that HCV core antigen might be a substitute for HCV RNA testing. Interestingly, the fluctuations in individual patients during a time course without any antiviral treatment were less pronounced with HCV core antigen vs the HCV RNA.
Furthermore, a significant lower correlation between HCV core antigen levels and HCV RNA levels is found in HBV coinfection patients with a r-value of 0.04, indicating that HCV core antigen actually may reveal additional information compared to HCV RNA levels, though this aspect has not been sufficiently explored yet.
The ability to commercialize an HCV antigen assay and need for a quantitative vs a qualitative assay will depend on the scenario, each of which will be addressed below in regard to where we stand and what we still need now: role in a diagnostic algorithm role in a blood bank/organ donor setting monitoring during natural history monitoring during and after therapy and predicting histological chances.
Who Should Get Tested For Hepatitis C
The CDC recommends that you get tested at least once no matter what. Definitely get screened if any of these things apply to you:
- You were born between 1945 and 1965.
- You use or inject drugs.
- You have ever injected drugs — even if it was just once or a long time ago.
- Youâre on kidney dialysis.
- You have abnormal alanine aminotransferase levels .
- You had a blood transfusion, blood components, or an organ transplant before July 1992.
- Youâve ever gotten clotting factor concentrates made before 1987.
- You received blood from a donor who later tested positive for hepatitis C virus.
- Youâre a health care worker, first responder, or have another job that exposes you to HCV-infected needles.
- You were born to a mother with HCV.
Recommended Reading: Hepatitis B And C Can Be Spread By
Testing For Hepatitis C
Two tests need to be done to discover if you have hepatitis C:
- Antibody test: Which establishes whether you have ever been exposed to the hepatitis C virus.
- PCR test: Which establishes whether the virus is still active and needs treating.
The two tests can often be done from one sample of blood which means you may only need to provide the sample once. Both tests can then be done on your sample at the laboratory. However, some services will perform one test and then call you back for a further blood sample to perform the second test.
Antibody test
A hepatitis C antibody test is the first test undertaken. This is to determine whether you have ever been exposed to the hepatitis C virus. It works by testing for the presence of antibodies to the virus generated by your immune system. If you receive a negative hepatitis C antibody test but have been experiencing symptoms or have been recently exposed to hepatitis C, then you are likely to be advised to have a second test.
It is important to remember that there is a ‘window period’. This is the short period of time when your immune system may not have had time to produce antibodies. It usually takes between six and twelve weeks for these antibodies to develop. However, in a few people it can take up to six months. So if you have the test within this window period and the result is negative, it does not necessarily mean that you don’t have the virus.
PCR test
Persons With Chronic Diseases
Refer to Immunization of Persons with Chronic Diseases in Part 3 for additional general information about vaccination of people with chronic diseases.
Chronic renal disease and patients on dialysis
People with chronic renal disease may respond sub-optimally to HB vaccine and experience more rapid decline of anti-HBs titres, and are therefore recommended immunization with a higher vaccine dose. Individuals undergoing chronic dialysis are also at increased risk for HB infection. In people with chronic renal disease anti-HBs titre should be evaluated annually and booster doses using a higher vaccine dose should be given as necessary.
Neurologic disorders
People with conditions such as autism spectrum disorders or demyelinating disorders should receive all routinely recommended immunizations, including HB-containing vaccine.
Chronic liver disease
HB immunization is recommended for non-immune persons with chronic liver disease, including those infected with hepatitis C, because they are at risk of more severe disease if infection occurs. Vaccination should be completed early in the course of the disease, as the immune response to vaccine is suboptimal in advanced liver disease. Post-immunization serologic testing may be used to confirm vaccine response.
Non-malignant hematologic disorders
Persons with bleeding disorders and other people receiving repeated infusions of blood or blood products are considered to be at higher risk of contracting HB and should be offered HB vaccine.
Don’t Miss: Hepatitis C Symptoms In Women
Hepatitis C Immune Globulin
Hepatitis B immune globulin has moved HBV-infected patients from the ranks of the untransplantable to ideal candidates for liver transplantation. The hope has long been that hepatitis C immune globulin will similarly ameliorate the impact of recurrence of HCV infection. Feray and colleagues demonstrated that HBIG containing anti-HCV reduced HCV reinfection in HBV/HCV-coinfected liver transplant recipients and conferred limited protection against de novo HCV infection.122 However, a randomized controlled study of HCIG in the prevention of posttransplant HCV infection found no benefit in terms of posttransplant clinical reinfection rate or HCV RNA levels.123 The availability, cost, and concerns about potential infectivity of pooled HCIG are likely to limit the impact of this therapy even if ultimately proved efficacious. A new monoclonal immune globulin directed at the envelope protein E2 and developed in the trimeric mouse model is currently in phase II trials. No preliminary results have been published to date for this preparation.
Hale Yarmohammadi, in, 2014
Hepatitis B Virus Antigens And Antibodies
The Structure of Hepatitis B Virus
The hepatitis B virus is a small DNA virus with unusual features similar to retroviruses, which is a prototype virus of the Hepadnaviridae family. HBV causes acute and chronic hepatitis in humans. The hepatitis B virus consists of an outer lipid envelope and an icosahedral nucleocapsid core composed of protein. The virus is one of the smallest enveloped animal viruses with a virion diameter of 42 nm, and also named Dane particles. Dane particles contains both envelope and core.
The outer envelope contains embedded proteins which are involved in viral binding of susceptible cells. There are three types of proteins: small hepatitis surface proteins, middle hepatitis surface proteins and large hepatitis surface proteins, they are totally composed of hepatitis B surface proteins. The nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse transcriptase activity.
There are three types of Hepatitis B Virus particles in infectious serum by electron microscopy, Dane particles, filamentous particles and spherical particles. Except for Dane particles , there also exist pleomorphic forms, as filamentous particles and spherical particles .
Hepatitis B Virus Antigens
Hepatitis B surface antigen HBsAg
Hepatitis B core antigen-HBcAg
Hepatitis B e antigen-HBeAg
The X gene codes for HBxAg. The product of the X gene is hepatitis B x antigen . It may be involved in carcinogenesis.
Hepatitis B Virus Antibodies
Referrence
Read Also: Hepatitis B And C Test
Role Of Hcv Core Antigen In Monitoring During And After Therapy
Monitoring HCV viral load with HCV core antigen during antiviral treatment might be an attractive tool for the future. Unfortunately data are too limited for strong recommendations thereof .1). Especially, there are no data with the newer antivirals available at present. For Pegylated Interferon plus Ribavirin regimens, there have been some studies suggesting that one can predict response as early as day 3, week 1 or week 2.
Taking A Hepatitis C Test
Hepatitis C testing is conducted on a sample of blood. Blood samples can be collected by a doctor, nurse, technician, or other health care provider from an adult patients vein using a small needle or a skin prick on a childs heel.
For an at-home hepatitis C test, patients collect a blood sample according to the manufacturers directions. Instructions provided in the test kit detail the steps to obtain a small sample of blood and mail it for testing.
Don’t Miss: Signs And Symptoms Of Hepatitis
Other Hepatitis C Tests
After an individual has received a reactive or positive result from a hepatitis C antibody test, they will need to have two follow-up tests.
The first test checks to see whether a person still has the virus the other measures the amount of the virus in the blood.
The first test is the hep C RNA qualitative test, also known as the PCR test. A positive result means that a person has the hepatitis C virus. A negative result means that the body has cleared the virus without treatment.
The second test is the hep C RNA quantitative test. The result of this test is given as a number rather than a positive or negative. This is because the test compares the amount of the virus in the body before, during, and after treatment.
The number given as a result of this test is known as the viral load. The lower amount of the hepatitis C virus in the blood, the better the chances that a person can eliminate the virus from their body.
After hepatitis C virus is diagnosed, other tests may be needed:
Certain behaviors, experiences, and medical procedures increase the risk of getting the hepatitis C virus, which is transmitted by contact with blood.
The following are risk factors for contracting the virus:
The Centers for Disease Control and Prevention advise all baby boomers get tested for hepatitis C. Baby boomers are people born between 1945 and 1965. They are five times more likely to have the virus than other adults.