Hepatitis C Antibody Without Pcr Reflex On Reactive Samples
- Hepatitis C Ab w/o PCR
- Lab Code
- Hepatitis C Antibody w/o PCR
- Description
-
The Qualitative detection of Hepatitis C virus IgG and IgM antibodies in human sera by the FDA approved Abbott ARCHITECT Anti-HCV test two-step chemiluminescent immunoassay.
In the first step, sample, assay diluent, and recombinant HCV antigen coated paramagnetic microparticles are combined. Anti-HCV present in the sample binds to the rHCV coated microparticles. In the second step, anti-human IgG/IgM acridinium-labeled conjugate is added, which binds to IgG and IgM anti-HCV. Then pre-trigger and trigger solutions are added to the reaction mixture. The resulting chemiluminescent reaction is measured as relative light units .
The presence or absence of IgG/IgM anti-HCV in the sample is determined by comparing the chemiluminescent signal in the reaction to the cutoff signal determined from an ARCHITECT Anti-HCV calibration. Specimens with signal to cutoff values 1.00 are considered reactive for IgG/IgM anti-HCV. Specimens with S/CO values < 0.79 are considered nonreactive and specimens with S/CO values between 0.80 and 0.99 are Indeterminate.
For anti-HCV testing with PCR reflex for REACTIVE results, see Hepatitis C Antibody with Reflex to PCR . Reactive anti-HCV will reflex to Hepatitis C RNA, Quantitative for confirmation with an additional charge.
- Synonyms
Who Should Get Tested For Hepatitis C
The CDC recommends that you get tested at least once no matter what. Definitely get screened if any of these things apply to you:
- You were born between 1945 and 1965.
- You use or inject drugs.
- You have ever injected drugs — even if it was just once or a long time ago.
- Youâre on kidney dialysis.
- You have abnormal alanine aminotransferase levels .
- You had a blood transfusion, blood components, or an organ transplant before July 1992.
- Youâve ever gotten clotting factor concentrates made before 1987.
- You received blood from a donor who later tested positive for hepatitis C virus.
- Youâre a health care worker, first responder, or have another job that exposes you to HCV-infected needles.
- You were born to a mother with HCV.
What Our Customers Say
- Thank you so much. I had alot of issues with getting results sooner and ARC point lab of Tampa got them in 48 hrs and the staff is very friendly and understanding. They were all very informative as well. Thanks ladies.
Taneisha Watson
Google ReviewNeeded a Covi-19/Sars test no more than 72 hours before departure to travel to France. Staff was very helpful, procedure painless and results in 15mn. Merci!
Aleth Voarick
Google ReviewThe test was comfortable , fast and the results came sooner than expected The staff and management very friendly and attentiveIf you having to take this test for traveling purposes this is the right place .
Antonio Kaik
Google Review
- The Food Sensitivity Test was easy to order, fast with results and the report was easy and simple to read. I would recommend this test to all of my family and friends. This was a very seamless and easy testing process.
Anthony Oliver
Facebook ReviewGreat experience at the Columbus, Ohio location! The staff was exceptionally friendly and so gentle and patient with my daughter who hates needles. I highly recommend them!
Emily Speltz West
Facebook ReviewARCpoint Labs around the country are known for their outstanding service with high integrity! The place to go if you are looking for workplace or judicial drug and alcohol testing, DNA testing and clinical testing services.
Gauri Bhalakia
Recommended Reading: Does Hepatitis C Go Away
Hepatitis C Reflex Testing
To ensure complete and timely diagnosis of HCV, HCV reflex testing is recommended following a reactive hepatitis C antibody screening test. Reflex testing means the laboratory will perform the hepatitis C antibody test, and if the result is positive, the laboratory will immediately perform an HCV RNA test on the same specimen. If the subsequent HCV RNA test is negative, HCV infection is effectively ruled out for most patients. If the reflex HCV RNA test is positive, a diagnosis of active HCV infection has been confirmed, and the individual should be referred directly for HCV care and treatment.
Reflex testing obviates the need for the patient to return for follow-up testing should the initially HCV antibody test be reactive. If the RNA test is negative, the work-up is done, and the patient may be reassured.
- Rationale for reflex testing:
What The Quantitative Results Mean

The quantitative test results indicate the exact amount of HCV in your blood. This number helps your doctor confirm whether you have a high or low viral load.
Measuring your viral load before treatment allows your doctor to monitor your viral load during and after treatment.
The viral load measurement doesnt indicate how severe your HCV infection or cirrhosis is. Your doctor will need to take a biopsy, or tissue sample, from your liver to learn more about how your liver has been affected by an HCV infection.
The viral load results from the quantitative PCR test can range from 15 to 100,000,000 IU/L.
If your results are:
- Fewer than 15 IU/mL: The virus is detected, but the amount cant be measured exactly. You may need to return later for another test to see if the measurement changes.
- Fewer than 800,000 IU/mL: A low viral load is detected.
- More than 800,000 IU/mL: A high viral load is detected.
- More than 100,000,000 IU/mL: The virus is detected and active infection is taking place.
- Inconclusive: HCV RNA cant be measured, and a new sample needs to be taken.
Recommended Reading: What Is Chronic Viral Hepatitis C
Discusses Conditions That May Cause Diagnostic Confusion Including Improper Specimen Collection And Handling Inappropriate Test Selection And Interfering Substances
A single negative hepatitis C virus RNA test result together with a reactive HCV antibody screen result with a signal-to-cutoff ratio of 8.0 or greater does not rule out the possibility of chronic HCV infection. Repeat testing for HCV RNA in 1 to 2 months is recommended in patient at risk for chronic hepatitis C.
Infants born to HCV-infected mothers may have false-reactive HCV antibody test results due to transplacental passage of maternal HCV IgG antibodies. HCV antibody testing is not recommended until at least 18 months of age in these infants.
Performance characteristics have not been established for the following types of serum specimen:
-Individuals under 10 years of age
-Grossly icteric
-Grossly lipemic
-Grossly hemolyzed
-Presence of particulate matter
Specific Hcv Rna Assays And Range Of Detectable Virus
HCV RNA tests use target amplification techniques. Several assays exist for HCV RNA testing. Methods include polymerase chain reaction , transcription mediated amplification , and branched chain DNA tests. Results are expressed as international units/mL . The different methods and different commercial assays each have a lower limit of quantification and lower limit of detection , therefore a patient’s results could be reported differently depending on the assay used. HCV RNA tests must have an LLOQ of 25 IU/mL or lower when used to assess treatment response with DAAs.
LLOQ = the lowest HCV RNA level that is within the linear and analytically acceptable range of the assay.
LLOD = the lowest level of HCV RNA that is detected 95% of the time.
Recommended Reading: How To Check For Hepatitis
Hepatitis C Ab With Reflex To Hcv Rna Qn Pcr
The Hepatitis C AB with reflex to HCV RNA, QN, PCR test contains 1 test with 2 biomarkers.
Hepatitis C AB with reflex to HCV RNA, QN, PCR
IMPORTANT – THIS IS A REFLEX TEST AND AN ADDITIONAL CHARGE OF $129 WILL BE APPLIED IF THE Hepatitis C Antibody is reactive.
If Hepatitis C Antibody is reactive, then Hepatitis C Viral RNA, Quantitative, Real-Time PCR will be performed at an additional charge of $129.00
For the detection of active HCV infection in HCV antibody positive individuals.
Clinical Significance
Hepatitis C Virus is a major cause of hepatitis. The clinical symptoms of an HCV infection are variable. Infection with HCV results in a chronic infection in 50 to 80% of cases. The “window” between HCV acquisition and seroreactivity is highly variable up to six months.
Tests After The Diagnosis
Once the doctor knows you have hep C, theyâll do tests to find out more about your condition. This will help determine your treatment. They could include:
- Genotype tests to find out which of the six kinds of hepatitis C you have.
- Liver function tests. They measure proteins and enzymes levels, which usually rise 7 to 8 weeks after youâre infected. As your liver gets damaged, enzymes leak into your bloodstream. But you can have normal enzyme levels and still have hepatitis C.
- Tests to check for liver damage. You might get:
- Elastography. Doctors use a special ultrasound machine to feel how stiff your liver is.
- Liver biopsy. The doctor inserts a needle into your liver to take a tiny piece to examine in the lab.
- Imaging tests. These use various methods to take pictures or show images of your insides. They include:
Don’t Miss: Hepatitis C And Liver Disease
Appropriate Uses Of The Hcv Rna Test
There are 4 major reasons that HCV RNA tests are used:
More rarely, HCV RNA is used when either very acute HCV infection is suspected or a false HCV Ab is suspected.
It would not be appropriate to repeatedly order HCV RNA viral load screening for a patient who is not on or was recently on HCV treatment, or to use the HCV viral load to determine the severity of the patient’s infection or the patient’s risk of developing significant liver disease.
Hepatitis C Antibody Blood Test With Reflex On Positives
Assess exposure to hepatitis C virus infection and tests blood safety.
Also Known As: HCV, Hep C.
Methodology: Immunoassay
Preparation: No fasting required. Stop biotin consumption at least 72 hours prior to the collection.
Test Results: 2-3 days. May take longer based on weather, holiday or lab delays.
Don’t Miss: Hepatitis B Surf Ab Quant
Discusses Physiology Pathophysiology And General Clinical Aspects As They Relate To A Laboratory Test
Hepatitis C virus is recognized as the cause of most cases of posttransfusion hepatitis and is a significant cause of morbidity and mortality worldwide. In the United States, HCV infection is quite common, with an estimated 2.4 million chronic HCV carriers.
Laboratory testing for HCV infection usually begins by screening for the presence of HCV antibodies in serum, using an FDA-approved screening test. Specimens that are repeatedly reactive by screening tests should be confirmed with HCV tests with higher specificity, such as direct detection of HCV RNA by reverse transcription-PCR or HCV-specific antibody confirmatory tests.
HCV antibodies are usually not detectable during the first 2 months following infection, but they are usually detectable by the late convalescent stage of infection. These antibodies do not neutralize the virus and they do not provide immunity against this viral infection. Decrease in the HCV antibody level in serum may occur after resolution of infection.
Current screening serologic tests to detect antibodies to HCV include enzyme immunoassay and chemiluminescence immunoassay . Despite the value of serologic tests to screen for HCV infection, several limitations of serologic testing exist:
-There may be a long delay between exposure to the virus and the development of a detectable HCV antibody
-False-reactive screening test result can occur
-A reactive screening test result does not distinguish between past and present HCV infection
Time For Processing Hcv Ab Test Results
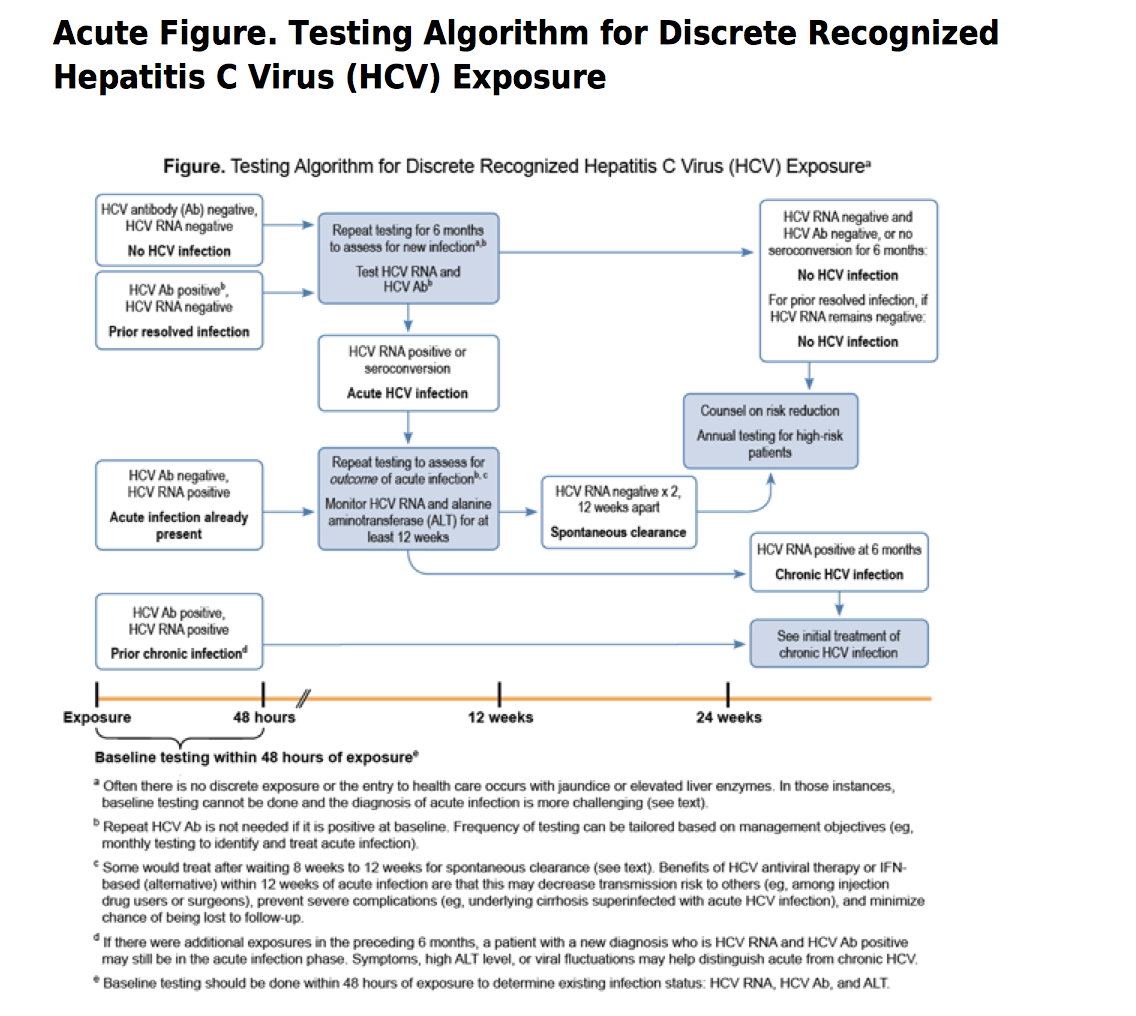
The turnaround time for 3rd-generation EIAs is at least 1 day. Many labs do not perform the tests on site and must send specimens to another lab for processing, which may further increase the turnaround time.
A point-of-care test is also available. The OraQuick® HCV Rapid Antibody Test is an FDA-approved test that can be performed with a fingerstick . It is also a CLIA-waived test and therefore can be used in clinic offices and outreach facilities. Results are reported as reactive or nonreactive within 20 minutes. Just as for the standard HCV Ab test done in the lab, a positive OraQuick® test must be confirmed by an HCV RNA test. The sensitivity and specificity of the test is similar to that of the laboratory-based assays.
Recommended Reading: Cirrhosis Caused By Hepatitis C
New Testing Options For Hepatitis C Virus
2017, Volume 27, Number 2
Danny L. Wiedbrauk, Ph.D., Scientific Director, Virology and Molecular Biology
Warde Medical Laboratory has a new testing option for diagnosing Hepatitis C Virus infections. The test, Hepatitis C Virus Antibody with Reflex to PCR includes HCV antibody testing with automatic referral to PCR for antibody-Reactive specimens. A final interpretation, based upon CDC guidelines, is provided for all specimens.
This testing protocol requires submission of two separate sample tubesone serum, the other plasma. The serum tube is tested for HCV antibody and if Reactive, the plasma tube is tested for HCV RNA by a quantitative PCR protocol.
Question 2 Why Are Hcv Rna Results Being Reported In Iu/ml
Results are reported in international units per milliliter to facilitate comparisons between results generated by different test methods. This is important because the various methods used by different laboratories are not standardized against each other. Use of IU/mL reporting units helps to make the comparison of viral load results across different methods more reliable.
Also Check: How Is Hepatitis C Spread
Taking A Hepatitis C Test
Hepatitis C testing is conducted on a sample of blood. Blood samples can be collected by a doctor, nurse, technician, or other health care provider from an adult patients vein using a small needle or a skin prick on a childs heel.
For an at-home hepatitis C test, patients collect a blood sample according to the manufacturers directions. Instructions provided in the test kit detail the steps to obtain a small sample of blood and mail it for testing.
How Much Does The Test Cost
The cost of hepatitis C testing depends on the tests that are performed, where the test is conducted, and a patients health insurance coverage. When testing is ordered by a doctor, patients with health insurance may find it helpful to discuss the cost of hepatitis C testing with their insurance company. In addition to the cost of testing, there may be other out-of-pocket costs such as copays and deductibles.
For patients without health insurance, or for whom insurance doesnt cover the cost of testing, it may be helpful to discuss the cost of hepatitis C testing with a doctor or hospital administrator.
At-home hepatitis C testing starts around $49. Some at-home kits test for multiple types of viral hepatitis at once, with the cost of these panels starting around $80.
Don’t Miss: Hepatitis A Symptoms And Treatment
What Does The Test Measure
Hepatitis C testing identifies antibodies to the hepatitis C virus, detects viral RNA, and/or determines the strain of hepatitis C. Hepatitis C testing may involve several different tests:
- Hepatitis C antibody test: Antibodies are a part of the bodys response to an infection. Testing for hepatitis C antibodies determines whether or not a patient has been exposed to the hepatitis C virus at some point in their life. If this test is positive, the next step is to test for hepatitis C RNA which can tell you if you have a current infection.
- Hepatitis C RNA test: RNA is a type of genetic material from the hepatitis C virus that can be detected in the blood. If test results are positive after a hepatitis C antibody test, doctors use a hepatitis C RNA test to look for and/or measure the amount of the virus in the blood. Qualitative HCV RNA tests can detect the presence of HCV RNA, while quantitative HCV RNA tests measure the amount of HCV RNA. Understanding the amount of HCV in the blood helps to monitor response to treatment.
- Genotype test: There are at least six types of hepatitis C, which are also called strains or genotypes. Treatment for hepatitis C depends on the strain, so genotype testing to guide treatment is performed in patients who are diagnosed with an HCV infection.
Are Test Results Accurate
Although no test is perfect, hepatitis C testing is an important and accepted method of testing for HCV. In order to reduce the risk of inaccurate results, doctors take steps to verify a patients diagnosis. For example, a positive test result for hepatitis C antibody requires confirmation with HCV RNA testing.
Also Check: How Can You Get Hepatitis A
Summary Of The Literature
For the all-adult review, the initial literature search yielded 4,867 studies. Twenty-nine duplicates were identified. Of 4,838 unique studies, 4,170 were deemed irrelevant by title/abstract screening, resulting in 668 full texts for review. Among these, 368 studies had data available to extract.
For the pregnancy review, the initial literature search yielded 1,500 studies. Two duplicates were identified. Of 1,498 unique studies, 1,412 were deemed irrelevant by title/abstract screening, resulting in 86 full texts for review.
The supplementary review yielded an additional 1,038 and 195 studies among all adults and pregnant women, respectively. Of these, 912 and 168 , respectively, were deemed irrelevant by title/abstract screening, resulting in 126 and 27 , respectively, full texts for review. One study was added to the pregnant women review outside of the formal literature search .
Considering all 104 applicable studies, the median anti-HCV positivity prevalence among all adults was 6.6% . Median anti-HCV positivity prevalence was 1.7% for the general population , 7.5% for ED patients , 3.3% for birth cohort members , 9.3% for others/multiple risk factors , 54.2% for persons who use drugs , 5.2% for persons with HIV or sexual risk , and 4.7% for immigrants . Considering 26 applicable studies among pregnant women, median anti-HCV positivity prevalence was 1.2% .