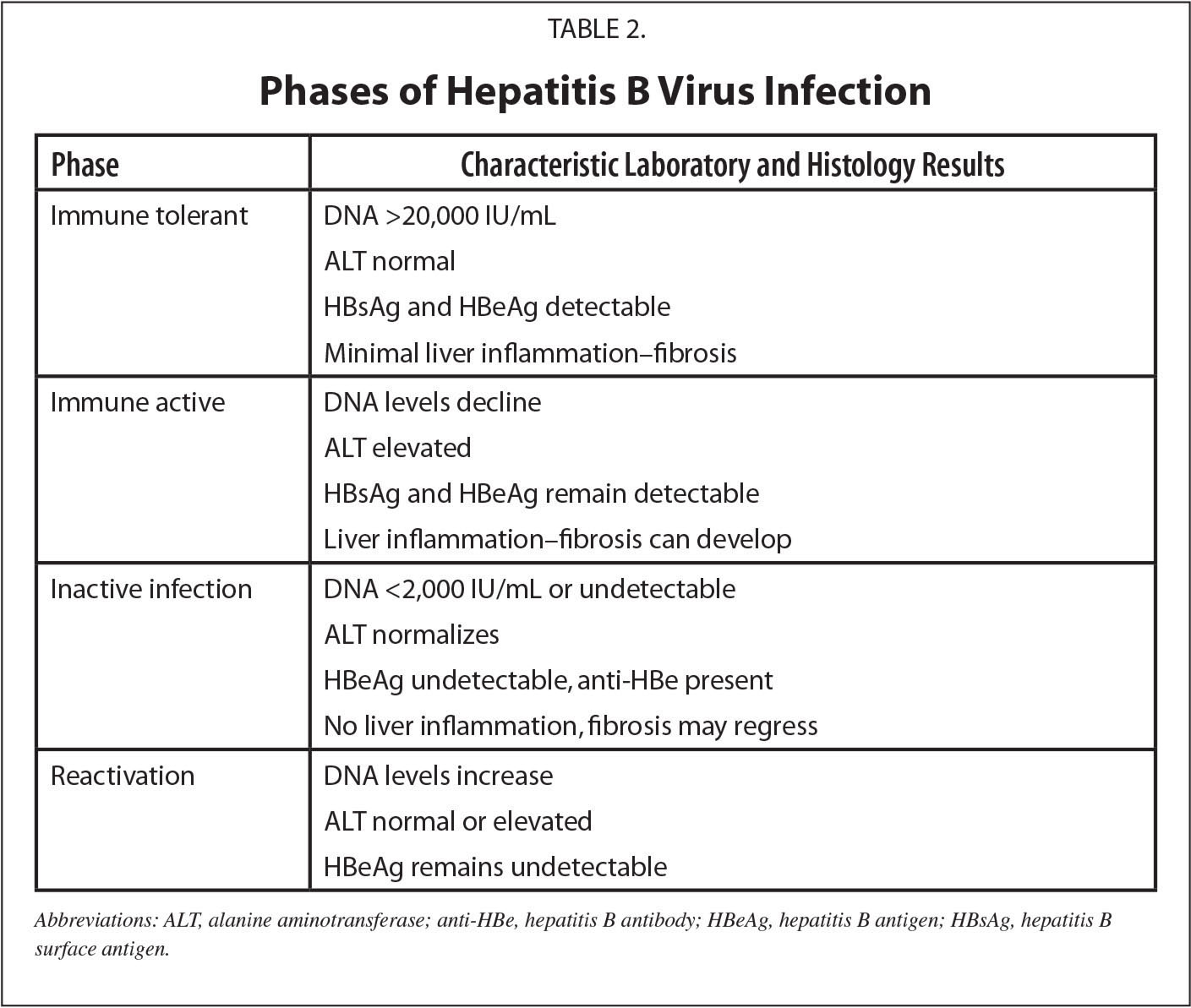
Immunogenicity And Vaccine Efficacy
More than 90% of infants, children, and adolescents and more than 90% of healthy adults younger than age 40 years develop a protective antibody response following a complete HepB vaccine series. However, there is an age-specific decline in immunogenicity. By 60 years, only 75% develop protective antibody titers. In adults receiving Heplisav-B, 90 to 100% develop adequate antibody after the 2-dose series.
Infants born to women who are HBsAg-positive are at high risk of HBV transmission and chronic HBV infection. HepB vaccination and 1 dose of HBIG administered within 24 hours after birth are 85% to 95% effective in preventing chronic HBV infection. HepB vaccine administered alone beginning within 24 hours after birth is 70% to 95% effective in preventing perinatal HBV infection.
HepB vaccine is 80% to 100% effective in preventing infection or clinical hepatitis in those who receive the complete vaccine series. Larger vaccine doses or an increased number of doses are required to induce protective antibody in most dialysis patients age 20 years or older and may also be necessary for other immunocompromised persons age 20 years or older. The recommended dosage of vaccine differs depending on the age of the recipient and type of vaccine.
For dialysis patients who did respond to vaccine, the need for booster doses should be assessed by annual testing of vaccine recipients for antibody levels, and a booster dose should be provided when antibody levels decline below 10 mIU/mL.
Ascletis Announces Dosing Of The First Patient In The Phase Iib Expansion Cohort Of Asc22 For Chronic Hepatitis B Functional Cure
— After the pre-Phase III clinical trial meeting with Center for Drug Evaluation of China National Medical Products Administration in June 2022, the pathway to the registration, including patient population, dose, treatment duration, etc. of ASC22 for functional cure of chronic hepatitis B has been agreed
— The Phase IIb Expansion Cohort will enroll 50 CHB patients with baseline HBsAg100 IU/mL who will be treated with 1.0 mg/kg ASC22 or placebo in combination with Nucleotide analogues for 24-week treatment plus 24-week follow-up
— The objective of this Expansion Cohort study is to confirm whether the rate of functional cure is similar to the data presented at oral session of the International Liver Congress 2022 held by the European Association for the Study of the Liver in June 2022, which showed that 42.9% of patients with baseline HBsAg 100 IU/mL obtained functional cure. The enrollment of 50 CHB patients is expected to be completed in early 2023
HANGZHOU and SHAOXING, China, Sept. 27, 2022 /PRNewswire/ — Ascletis Pharma Inc. today announces dosing of the first patient in the Phase IIb Expansion Cohort of subcutaneously administered PD-L1 antibody ASC22 for functional cure of chronic hepatitis B .
Lim J K, Nguyen M H, Kim W R, et al. Prevalence of Chronic Hepatitis B Virus Infection in the United States . The American journal of gastroenterology 2020, 115: 1429-38.
About ASC22
About Ascletis
For more information, please visit www.ascletis.com.
Phase 3 Study Of Gsk548470 In Patients With Compensated Chronic Hepatitis B With Poor Response To Other Drugs
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| First Posted : November 21, 2011Results First Posted : November 3, 2013Last Update Posted : June 22, 2015 |
| Drug: GSK548470 300 mg tablet | Phase 3 |
| Study Type : | |
| Treatment | |
| Official Title: | A Multi-center, Open-label Study of GSK548470 in Patients With Compensated Chronic Hepatitis B With Poor Response to Other Drugs |
| Study Start Date : |
| GSK548470 300 mg tablet is administered orally once daily | Drug: GSK548470 300 mg tabletBlue tablets containing 300 mg of tenofovir disoproxil fumarateOther Name: GSK548470 |
Don’t Miss: Is There An Immunization For Hepatitis C
Challenges In Data Diversity
With hepatitis B disproportionately affecting people in Asian countries, it is logical that a vast majority of such trials are conducted in the region, particularly in China. But Seto asks: Can the FDA accept predominantly Asian data?
Indeed, according to GlobalDatas Clinical Trial Database, most of the planned and ongoing hepatitis B clinical trials are being conducted in China. GlobalData is the parent company of Clinical Trials Arena.
The FDA has previously rejected data purely from China as there are questions if it would be representative of the US population. To bolster patient diversity, all sponsors should, alongside in Western countries, recruit from sub-Saharan Africa and Latin America, Seto notes.
The hunt for a functional cure in HBV has been elusive. While HBV vaccines offer as much as 90100% protection from an infection, positive treatment data is harder to achieve. There is significant unmet need, with the burden of hepatitis B affecting nearly 300 million people globally with 1.5 million infections each year, according to the World Health Organizations 2019 data.
Experts are optimistic, noting there is the possibility that there will be a regimen that could offer functional cure in the next few years. But they are also cautious: while these are exciting times, theres still a lot of work to be done.
Takeaways:
Related Companies
Genetically Engineered T Cells
To restore adequate HBV-specific T cell immunity, the adoptive transfer of genetically engineered T cells can be a promising strategy . T cells separated from the peripheral blood of patients with CHB were expanded and activated in vitro. The expanded T cells were engineered to encode HBV-specific T cell receptors by using viral vectors and then were reinfused to patients with CHB. This approach could be achieved through T-cell receptor gene transfer or the use of chimeric antigen receptor T cells . TCR-redirected HBV-specific T cells were able to recognize HBV-infected cells in an in vitro study . T cells with a CAR specific for HBeAg were also reported to localize to the liver of mice to reduce the replication of HBV with only transient liver damage .
Don’t Miss: What Should I Do If I Have Hepatitis B
Is Cure Coming In Future
I saw you on YouTube that you are participating in clinical trails for cure hepatitis b.
I saw some trails going to made for hepatitis b cure.When is nasvac therapeutic vaccine is going to be released?How many phases of clinical trails will be there?Why is thervacb show so much promising on curing hepatitis b?Is that mouse model is successful in any previous trails of other therapeutic vaccines?
Im like the most people who live with hope
Dear Helpme786,
Im a senior HBV virologist and director of a local drug discovery institute. Your questions about a cure coming are very good. The good news is that there are an enormous number of drug and vaccine strategies being tried in Universities, small biotechnology companies, and major pharmaceutical companies. I am personally confident that major improvements in therapy for HBV will arrive within 5 years, or perhaps a bit longer. However, please remember that this is just my personal opinion, and that it is impossible to predict the pace of scientific and medical advances. We are working at the edge of the unknown, and the complexity of the problem is truly daunting.
As to your specific questions:
I hope this helps.
My last question : under taking medications can I live my normal life and going to my job Im working in a small software company in night shifts.
Thank you doctor
Thanks for your questions and thanks @john.tavis for your informative answers.
Thomas
Today i came with new question .
Dear Helpmegod,
John Tavis
Bulevirtide Beneficial In Hepatitis D: Phase 3 Data
Sara Freeman
LONDON Bulevirtide may not just treat but perhaps be a potential cure for hepatitis D in some patients, as was suggested at the annual International Liver Congress.
Data from an ongoing phase 3 trial showed that, after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable hepatitis delta virus RNA levels and normalized ALT levels.
Dr Heiner Wedemeyer
The good message for our patients is that the initial data of the smaller phase 2 trials will really be confirmed, so the drug works, Heiner Wedemeyer, MD, said at a media briefing ahead of his presentation at the meeting sponsored by the European Association for the Study of the Liver.
It induces a decline in viral load and, very importantly for us as hepatologists, liver enzymes normalize, this is really good news added Wedemeyer, who is the clinic director of the department of gastroenterology, hepatology, and endocrinology at Hannover Medical School.
This is really an almost historic moment for hepatology, he said. Its the first time that these patients have an antiviral treatment they are afraid of dying and now they have a hope.
Dr Thomas Berg
Giving his thoughts, Thomas Berg, MD, Secretary General of EASL, said: We are entering into a golden age of hepatology science when it comes to viral hepatitis.
Don’t Miss: How To Treat Hepatitis C
Targets For An Hbv Cure
Several drugs are now under development that directly target the HBV replication cycle or enhance the human immune response . New drugs for HBV cure include agents that directly target virus life cycle or those that indirectly modulate host factor/host immune response .
Targets of hepatitis B virus replication in hepatocytes. HBV, hepatitis B virus NTCP, Na taurocholate co-transporting polypeptide rcDNA, relaxed circular DNA cccDNA, covalently closed circular DNA pgRNA, pregenomic RNA CpAMs, core protein assembly modulators NAs, nucleoside analogues.
Clearing Acute Hepatitis B
Some studies suggest that up to 95% of adults with acute HBV infection will spontaneously clear the virus, usually within six months, with no lasting repercussions.
Chronic hepatitis B occurs when the immune system does not clear the virus. Around one of every 20 people acutely infected with HBV will progress to this persistent stage of infection.
Chronic hepatitis B is a slowly progressive disease in which ongoing inflammation causes the gradual scarring of the liver. This can lead to cirrhosis and hepatocellular carcinoma .
However, the course of chronic HBV infection is not set. Some people may progress faster than others, while others may never develop overt symptoms.
Statistically speaking:
- The risk of cirrhosis in people with chronic hepatitis B is approximately 10% to 20% over 20 years, increasing to 40% after 30 years.
- The risk of hepatocellular carcinoma increases by 2% and 3% per year in people with HBV and cirrhosis. People without cirrhosis can also get it, but the annual risk drops to around 0.02%.
Recommended Reading: Can You Live With Hepatitis C
Secular Trends In The United States
Hepatitis B became nationally notifiable as a distinct entity during the 1970s after serologic tests to differentiate different types of hepatitis became widely available.
In 2018, a total of 3,322 cases of acute hepatitis B were reported to CDC, for an overall incidence rate of 1.0 cases per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 21,600 acute hepatitis B cases occurred in 2018. The rate of reported acute HBV infections declined approximately 90% since recommendations for HepB vaccination were first issued, from 9.6 cases per 100,000 population in 1982 to 1.0 cases per 100,000 population in 2018.
During 2009 through 2013, the combined incidence of acute HBV infection in three states increased 114% and was associated with increasing injection-drug use. Incidence is greatest for persons age 40 through 49 years persons age 19 years or younger have the lowest incidence , likely a result of routine infant vaccination.
Although HBV infection is uncommon among adults in the general population , it is highly prevalent in certain groups. Generally, the highest risk for HBV infection is associated with lifestyles, occupations, or environments in which contact with blood from infected persons is frequent. Chronic HBV infection has been identified in 3.5% to 20.0% of persons who inject drugs in a variety of settings, and 22.6% of PWID have evidence of past infection.
Phase Iib Hepatitis B Data Has Caveats
GSK, formerly GlaxoSmithKline, presented positive interim analysis at this years EASL Liver Congress from the Phase IIb B-clear trial investigating bepirovirsen in treatment-naïve patients and ones already taking nucleotide analogues . In the trials secondary endpoint, 28% of patients on NA and 29% of treatment-naïve participants experienced lowered HBsAg and hepatitis B virus levels with 300mg bepirovirsen at the end of the 24-week treatment. NAs suppress HBV to undetectable levels but must be taken long term, if not indefinitely.
Final B-clear data is expected later this year. Phase IIb investigator Dr Man-Fung Yuen notes the assets potential for durable efficacy at 48 weeks after the first dose, which is the trials primary endpoint, will be a key efficacy indicator.
Bepirovirsen is likely to have some lingering efficacy impact after the therapy is stopped, notes Yuen, who is also a hepatology professor at the University of Hong Kong. I would be comfortable to say that the sustained effects would last for at least six months after stopping the therapy, he says.
In a Phase IIa trial , after two weeks but participants received a lower dose than in the Phase IIb. The best performing Phase IIa cohort in terms of durability was given the drug once weekly at 120mg for 12 weeks. In Phase IIb, the positive data was from patients given a 300mg weekly dose at 24 weeks.
Don’t Miss: Hepatitis B Core Ab Total Reactive
Immune Modulatory Therapies Or Indirect Antivirals
A weak innate and HBV-specific immunologic response occurs in patients with CHB . Chronic HBV infection leads to T-cell dysfunction , a state characterized by poor effector cytotoxic activity, impaired cytokine production, and the sustained expression of multiple inhibitory receptors . Thus, the identification of immunomodulatory targets is important in therapeutic strategies aimed at restoring HBV-specific immune responses with immunomodulatory agents. However, the results achieved thus far with immune modulators have been disappointing.
3.2.1. Toll-Like Receptor Agonists
3.2.2. Engineered T Cells
The adoptive transfer of newly engineered HBV-specific T cells may be a novel strategy . Genetic reprogramming to create functional T cells to eliminate HBV-infected hepatocytes could be achieved through T-cell receptor gene transfer or through the use of chimeric antigen receptor T cells . Tests of HBV-specific T cells with CAR or classic TCRs both in vitro and in HBV transgenic mice have shown selective elimination of HBV-infected cell lines and control of HBV replication with only transient liver damage, respectively .
3.2.3. Immune Checkpoint Inhibitors
3.2.4. Therapeutic Vaccine
Hepatitis B: Can Gsks Bepirovirsen Deliver Functional Cure
GSKs antisense oligonucleotide is looking to further prove its value in disrupting viral replication via a Phase III test.
Last month, GSK provided an early look at positive Phase IIb data that got the hepatitis B field excited. Results show that bepirovirsen could potentially lead to functional cure, which means that the candidate lowered hepatitis B surface antigen to undetectable levels.
The Phase IIb data is a major breakthrough, says Dr Pietro Lampertico, professor of gastroenterology at the University of Milan. This is the first time in a decade whereby a new candidate was able to show lowered HBsAg to undetectable levels after weeks of treatment, Lampertico explains.
With GSK gearing to initiate a Phase III bepirovirsen trial in the first half of 2023, this is the first time an antisense oligonucleotide candidate would be investigated at this stage. Its also been years since a new hepatitis B agent would be studied at Phase III, notes Dr Jordan Feld, senior scientist at Toronto General Hospital Research Institute.
As such, all eyes are on GSK on how it will design its forthcoming study, and if the assets efficacy profile sustains in the next development stage.
You May Like: Hepatitis B Or C Symptoms
Advances In New Drugs To For Curing Hepatitis B And Hepatitis D Announced At Ilc 2021
For Immediate Release
Media Release
Advances in new drugs to for curing Hepatitis B and Hepatitis D announced at ILC 2021
Thursday 24 June 2021 Leading hepatology researchers announced important new developments in hepatitis research at the International Liver Congress 2021 today. This includes new data on antivirals to cure Hepatitis B and Hepatitis D and the application of infusion chemotherapy with P-1 inhibitors to treat liver cancer.
Other announcements included a review of the impact of the COVID-19 pandemic on efforts to eliminate Hepatitis C in the USA and some encouraging data from a trial of a new liver dialysis device to treat acute on chronic liver failure .
Scientists and advocates have long argued that if we are realistically going to eliminate Hepatitis B, then we will need a functional cure, said Philip Newsome, Secretary General of EASL and Professor of Experimental Hepatology and Director of the Centre for Liver Research at the University of Birmingham in the UK. The results from the trial of RNAi therapeutic drug VIR-2218 are an encouraging example that a cure is possible sooner than later with potential real-world implications for the 300 million people living with the disease.
Todays official press conference highlighted five studies covering treatment and cure research for hepatitis and acute on chronic liver failure selected from over 1500 abstracts being presented at ILC 2021.
Impact of COVID-19 on eliminating Hepatitis in the U.S.
Contact:
European Commission And Thervacb Join Forces
The role of viral hepatitis as a public health threat has long been underestimated. Only very recently, the United Nations in their 2030 Agenda for Sustainable Development called for international action to combat viral hepatitis and reduce the disease burden. The major killer is the hepatitis B virus causing liver cirrhosis and liver cancer. Worldwide 880,000 humans die each year from the consequences of an HBV infection.
A prophylactic vaccine is available to prevent HBV infection, but more than 3% of the worlds population are chronically infected and do not profit from that vaccine anymore. For those suffering from chronic hepatitis B, until today no curative treatment option exists.
The European Commission therefore selected the project TherVacB led by Helmholtz Zentrum München for a five-year funding within the Horizon 2020 program. A consortium of leading virologists, immunologists and physicians specialized in treating viral hepatitis, will use a newly designed therapeutic vaccine, TherVacB, as an immunotherapy to cure HBV. TherVacB will be evaluated in a three-year clinical trial starting in 2022 conducted in Europe and in Africa. Integration of a partner site in Tanzania shall help building local capacities for diagnosing and treating hepatitis B and support an important goal of the consortium to raise awareness for hepatitis B.
Recommended Reading: What Are The 5 Types Of Hepatitis