Costs Health Utilities And Mortality Rates
contains all cost values and utility values for health states. Direct program costs and direct medical costs are included. All final cost figures were converted to 2010 U.S. dollars using the U.S. Bureau of Labor Statistics Consumer Price Index . The Supplementary Data describes the calculation of other-cause mortality rates for people with diabetes.
Guidance On Reporting Adverse Events Following Immunization
Vaccine providers are asked to report, through local public health officials, any serious or unexpected adverse event temporally related to vaccination. An unexpected AEFI is an event that is not listed in available product information but may be due to the immunization, or a change in the frequency of a known AEFI.
Refer to Reporting Adverse Events Following Immunization in Canada and Adverse events following immunization in Part 2 for additional information about AEFI reporting.
Characteristics Of The Pregnant Women And Their Infants
A total of 992 HBsAg positive pregnant women who delivered in 13 hospitals located in the two counties between May 1, 2018, and October 31, 2019. Among them, 604 were interviewed when upon arrival at the hospital for delivery, and a total of 619 infants were born, with 511 receiving three doses of 20 g HepB between one and six months apart. The blood samples of 319 infants were collected one month later, after they had completed three doses of vaccine, to test for HBsAg and HBsAb. In order to estimate the HBsAb variation of infants, blood samples of 398 infants, who had been vaccinated with 20 g CHO HepB, were collected in June 2020, which included 287 infants who had their sero-samples collected for the first time. In total, 419 HBsAg-positive women and their 430 infants were analyzed . The average age of the women was 29.6±4.3 years, with the oldest and youngest women being 47 and 19 years old, respectively. Those with college education or above accounted for 54.9% . The oldest child was 24 months old, while the youngest child was seven months old. Ethnic Han women accounted for 99.1% for the total, whereas female farmers for 24.1% . Among the women, 21.5% were aware that they were infected with HBV in the previous year. Furthermore, 27.4% of them had received antiviral therapy during pregnancy . Twenty-five of the pregnant women gave birth before 37 weeks, and 14 out of the 270 pregnant women who filled out ALT values had ALT values higher than 40.
Figure 1
Recommended Reading: Can Your Body Cure Hepatitis C
Hepatitis B Vaccine On The Nhs
A hepatitis B-containing vaccine is provided for all babies born in the UK on or after 1 August 2017. This is given as part of the 6-in-1 vaccine.
Hospitals, GP surgeries and sexual health or GUM clinics usually provide the hepatitis B vaccination free of charge for anyone at risk of infection.
GPs are not obliged to provide the hepatitis B vaccine on the NHS if you’re not thought to be at risk.
GPs may charge for the hepatitis B vaccine if you want it as a travel vaccine, or they may refer you to a travel clinic for a private vaccination. The current cost of the vaccine is around £50 a dose.
What Are The Treatment Options For Hepatitis B
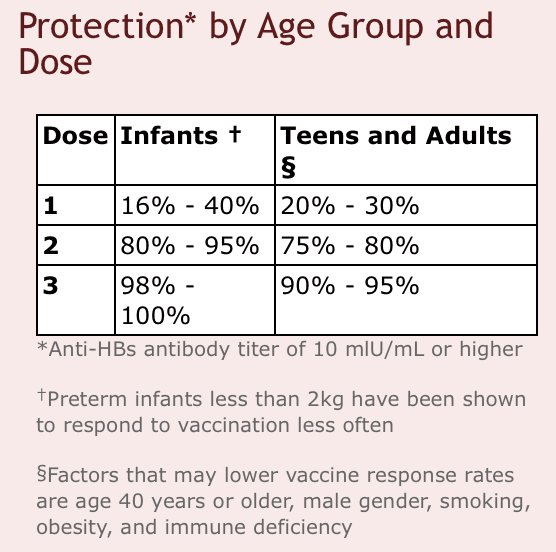
Currently, there is no specific antiviral treatment recommended for persons with acute hepatitis B disease as approximately 95% of infected immunocompetent adults recover spontaneously. Specific treatment is available to support people with chronic HBV infection. The main goal of the available care is to maintain comfort, relieve symptoms, and prevent patients from passing the infection to others. However, notably, not all patients with chronic HBV need to be on medication . Patients with active signs of liver disease may benefit the most from current treatment. The Food and Drug Administration has approved for the treatment of chronic hepatitis B interferon- and oral antiviral agents . Present treatment for chronic hepatitis B can slow or prevent the progression of cirrhosis, reduce the incidence of liver cancer, and improve long term survival and quality of life, but are not curative. Therefore, most people who start hepatitis B treatment must continue for life. The side effects of the therapies and required regular monitoring increases the difficulty and complexity of patient management. Hence, hepatitis B vaccination is plan A in the fight against hepatitis B. Vaccination is, compared to other interventions, an economically attractive option, both in terms of cost-effectiveness and benefit-cost ratios .
You May Like: Can You Cure Hepatitis B
Contraindications And Precautions To Vaccination
As with other vaccines, a history of a severe allergic reaction to a vaccine component or following a prior dose is a contraindication to further doses. Moderate or severe acute illness in a patient is considered a precaution to vaccination, although persons with minor illness may be vaccinated.
In 2011, the Institute of Medicine concluded that the evidence convincingly supports a causal relationship between HepB vaccine and anaphylaxis in yeast-sensitive persons. HepB vaccination is contraindicated for persons with a history of hypersensitivity to yeast or any other vaccine component. The estimated incidence of anaphylaxis among HepB vaccine recipients is 1.1 per million vaccine doses administered.
Some presentations of HepB vaccines contain latex, which may cause allergic reactions.
Vaccination is not contraindicated in persons with a history of multiple sclerosis, Guillain-Barré syndrome, autoimmune disease or other chronic diseases.
Contraindications to combination vaccines that contain HepB vaccine include the contraindications to the individual component vaccines specific ingredients differ by vaccine.
Hepatitis B Vaccine: Canadian Immunization Guide
For health professionals
Last partial content update : May 2022
The footnotes in and the accompanying text description for the figure have been revised to align with the corresponding figure in Protocole d’immunisation du Québec, 5e édition from which it was adapted.
Last complete chapter revision :
Read Also: Hepatitis C And Liver Disease
What Is The Morphology Of Hbv
HBV is an oncogenic DNA virus that belongs to the Hepadnaviridae family. The discovery of the etiologic agent of hepatitis B remains a remarkable scientific achievement. It was discovered in 1965 by Dr Blumberg, who won the Nobel Prize in Medicine for his discovery in 1976 . HBV virus, initially called the Dane particle, is a 42-nm virus . HBV is composed of a nucleocapsid core, surrounded by an outer lipoprotein coat . The virus contains 3 primary structural antigens: surface , core , and e . HBsAg is produced in excess amounts and found in the blood of infected individuals in the form of spherical and tubular particles . These immunogenic, but noninfectious, subviral particles lack genomic DNA and paved the way to develop hepatitis B vaccines . HBV is divided into 4 major phenotypic subtypes based on antigenic epitopes presented on its envelope proteins, and comprises 10 major genotypes that differ at the nucleotide level across full-length genotypes by> 8% . The HBV genotypes have distinct virological characteristics and geographical distributions however, the licensed HBV vaccines are effective against all genotypes .
A, Electron micrograph of hepatitis B virus : Dane particles and spherical and tubular surface antigen particles . Source: Centers for Disease Control and Prevention. As a work of the U.S. federal government. B, A simplified figure of the HBV particle and surface antigens.
The Safety Of Hepatitis B Vaccination Programs
Numerous clinical trials and widespread practical applications have demonstrated that hepatitis B vaccines are very safe. Since 1982, over 1 billion doses of hepatitis B vaccine have been used worldwide. Adverse events after immunization against hepatitis B are infrequent and generally mild and transient. Except for localized pain, placebo-controlled studies have revealed that reported events occur no more frequently among vaccinees than among persons receiving placebo . Data from numerous long-term studies fail to causally link other serious adverse events to hepatitis B vaccination. Data do not indicate a causal association between hepatitis B vaccine and neurological disease , leukemia, diabetes mellitus, demyelinating disorders, chronic fatigue syndrome, arthritis, autoimmune disorders, asthma, hair loss, or sudden infant death syndrome. The Global Advisory Committee on Vaccine Safety has confirmed the excellent safety profile of hepatitis B vaccine and continues to monitor the safety of this vaccine. Furthermore, hepatitis B vaccination can be administered safely to pregnant women during any trimester of pregnancy and to breastfeeding women. Both low birth weight and premature infants and HIV-positive persons can receive hepatitis B vaccination. Hepatitis B vaccination is contraindicated only for persons with a history of allergic reactions to yeast or any of the vaccines components.
Don’t Miss: Where To Get Tested For Hepatitis B
Comparison Of As02 And As04
In summary, a three-dose primary course of the adjuvanted HBV vaccine HB-AS02 induces more rapid, enhanced, and persistent protection in pre-dialysis, peritoneal dialysis, and hemodialysis patients than a four-dose primary course of HB-AS04 , an adjuvanted HBV vaccine licensed in Europe for use in patients with renal insufficiency . The higher postvaccination GMCs for anti-HBs antibodies and the greater proportion of subjects achieving anti-HBs antibody concentrations of X100mIU/mL after vaccination with HB-AS02 suggest that duration of protection is also likely to be enhanced, potentially affording further reductions in the need for booster doses in this at-risk population. Such adjuvanted hepatitis B vaccines may also have clinical utility in other categories of immunocompromised patients .
Reviewhepatitis B Vaccination: The Key Towards Elimination And Eradication Of Hepatitis B
Hepatitis B virus infection is a global health problem. Worldwide, about 360 million people are chronically infected with the virus. They continue to spread the virus to others and are themselves at risk of chronic liver diseases and hepatocellular carcinoma. The infection can now be treated by antivirals or interferons and the transmission route can be interrupted. Nevertheless, the most effective means is to immunize all susceptible individuals, especially young children, with safe and efficacious vaccines. The combined efforts of vaccination, effective treatment and interruption of transmission make elimination of the infection plausible and may eventually lead to eradication of the virus. Because hepatitis B vaccination has a key role in the control of hepatitis B, properties of this vaccine, its effectiveness in pre-exposure and post-exposure settings, duration of protection after vaccination and the need of booster doses are discussed. Mass hepatitis B vaccination in children decreases the carriage of the virus, and the diseases associated with acute and chronic infection, including hepatocellular carcinoma. Challenges that need to be solved to expand mass vaccination, and the strategies towards elimination and eventual eradication of hepatitis B in the world are also discussed.
Recommended Reading: How Many Hepatitis Are There
Observational Study Of Vaccine Efficacy 24 Years After The Start Of Hepatitis B Vaccination In Two Gambian Villages: No Need For A Booster Dose
-
Current address: International Agency for Research on Cancer, Lyon, France
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Current address: Harvard University School of Public Health, Boston, Massachusetts, United States of America
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation Medical Research Council Laboratories, The Gambia, Banjul, the Gambia, West Africa
-
Affiliation MRC Keneba, MRC Unit The Gambia, Banjul, The Gambia
-
Affiliation London School of Hygiene and Tropical Medicine, London, United Kingdom
Chronic Hepatitis C Infection
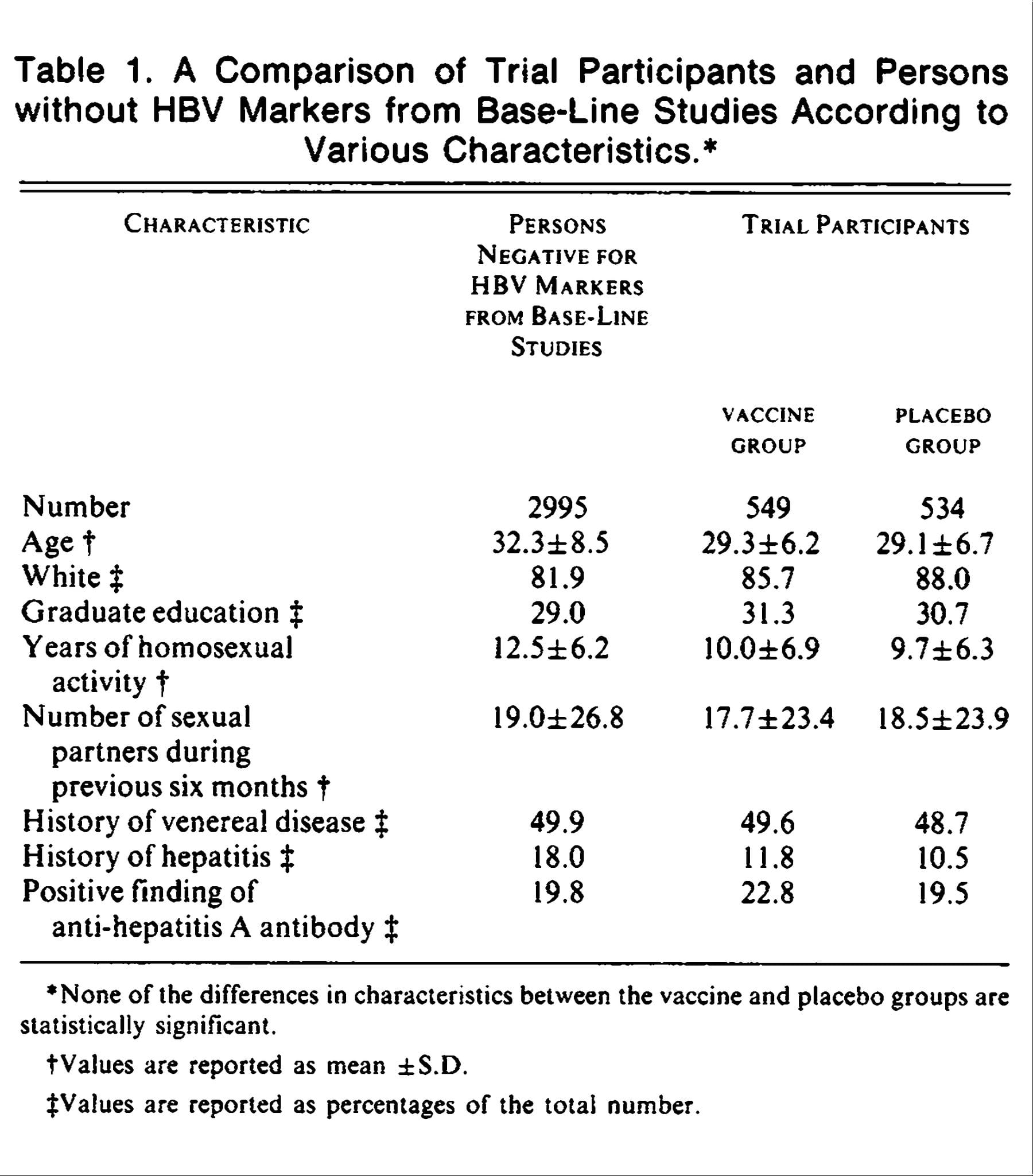
The major decrease in chronic hepatitis B virus infection because of infant vaccination has been accompanied by a substantial decrease in chronic hepatitis C virus infection, probably due to improved sterile precautions in medical use of needles. Among the controls in a casecontrol study of adults in The Gambia there was a substantial prevalence of HCV among those born before the 1950s but a much lower prevalence among those born later . In the present survey of young adults born during 19861990 the prevalence of HCV was again only 0.5% , confirming the low HCV prevalence in recent generations. The present study tested much larger numbers of Gambians than any previous study and involved a representative community sample. Comparison of the results among older adults in other studies with those among young adults in the present study suggests a birth cohort effect involving almost a ten-fold decrease in the prevalence of HCV in The Gambia. This suggests that, in The Gambia as in many other countries, the mid-century epidemic of iatrogenic HCV infection from unsterilized needles has been controlled by the sterile procedures of the last quarter of the century. So, although HBV and HCV are causes of almost all liver cancer and cirrhosis deaths today in The Gambia, both should largely vanish over the coming decades.
Read Also: What To Do When You Have Hepatitis B
Safety And Efficacy Of Hepatitis B Vaccination In Cirrhosis Of Liver
Rama Mohan Pathapati
Abstract
Introduction. Patients with chronic liver disease are more likely to have severe morbidity and fatality rate due to superimposed acute or chronic hepatitis B infection. The literature has shown that hepatitis B vaccines are safe and effective in patients with CLD, but the data in cirrhosis liver is lacking. We assessed the safety and immunogenicity of HBV vaccine in patients with cirrhosis liver. Methods. CTP classes A and B CLD patients negative for hepatitis B surface antigen and antibody to hepatitis B core antigen were included. All patients received three doses of hepatitis B vaccine 20mcg intramuscularly at 0, 30, and 60 days. Anti-HBs antibody was measured after 120 days. Results. 52 patients with mean age years were studied. Response rates in CTP classes A and B were 88% and 33.3%. We observed that the alcoholic chronic liver disease had less antibody response than other causes of chronic liver disease such as cryptogenic 69% and HCV 75%. . Patients with cirrhosis liver will have low antibody hepatitis B titers compared to general population. As the age and liver disease progress, the response rate for hepatitis B vaccination will still remain to be weaker.
1. Introduction
2. Methods
2.1. Statistical Analysis
3. Results
| ALD: alcoholic liver disease CR: cryptogenic hepatitis HCV: hepatitis C virus BC: budd chiarri AI: autoimmune hepatitis and WD: wilsons Disease. |
4. Discussion
5. Study Limitations
6. Conclusions
References
Emergency Hepatitis B Vaccination
If you have been exposed to the hepatitis B virus and have not been vaccinated before, you should get immediate medical advice, as you may benefit from having the hepatitis B vaccine.
In some situations, you may also need to have an injection of antibodies, called specific hepatitis B immunoglobulin , along with the hepatitis B vaccine.
HBIG should ideally be given within 48 hours, but you can still have it up to a week after exposure.
Also Check: What Organ Does Hepatitis Affect
The Hbv Infection Status And Information Of Hbsag
Pregnant women were screened for HBsAg using the ELISA method when they visited the hospital for perinatal health care in China. All the pregnant women who participated in the survey were interviewed and given a questionnaire to fill out. Personal information about the pregnant women, such as age, gestational age, history of therapy, and other factors, were included in the questionnaire. Before delivery, 5 ml of blood sample was taken from the expecting mothers. All serum samples were delivered to the Henan provincial Center for Disease Control and Prevention to test for HBsAg, HBeAg, and HBeAb using ELISA test kits . The amount of HBV DNA in the collected serum was measured by real-time PCR using the HBV DNA test kit .
Secular Trends In The United States
Hepatitis B became nationally notifiable as a distinct entity during the 1970s after serologic tests to differentiate different types of hepatitis became widely available.
In 2018, a total of 3,322 cases of acute hepatitis B were reported to CDC, for an overall incidence rate of 1.0 cases per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 21,600 acute hepatitis B cases occurred in 2018. The rate of reported acute HBV infections declined approximately 90% since recommendations for HepB vaccination were first issued, from 9.6 cases per 100,000 population in 1982 to 1.0 cases per 100,000 population in 2018.
During 2009 through 2013, the combined incidence of acute HBV infection in three states increased 114% and was associated with increasing injection-drug use. Incidence is greatest for persons age 40 through 49 years persons age 19 years or younger have the lowest incidence , likely a result of routine infant vaccination.
Although HBV infection is uncommon among adults in the general population , it is highly prevalent in certain groups. Generally, the highest risk for HBV infection is associated with lifestyles, occupations, or environments in which contact with blood from infected persons is frequent. Chronic HBV infection has been identified in 3.5% to 20.0% of persons who inject drugs in a variety of settings, and 22.6% of PWID have evidence of past infection.
Don’t Miss: How Can You Catch Hepatitis B
Postexposure Management Of Health Care Personnel After Occupational Exposure To Blood And Body Fluids By Health Care Personnel Hepb Vaccination And Response Status
| HepB Vaccination and Response Status | Postexposure testing results for source patient | Postexposure testing results for HCP | HBIG* postexposure prophylaxis |
|---|---|---|---|
| Complete vaccination | Yes |
*HBIG should be administered intramuscularly as soon as possible after exposure when indicated. The effectiveness of HBIG when administered greater than 7 days after percutaneous, mucosal, or nonintact skin exposures is unknown. HBIG and HepB vaccine should be administered in separate anatomic injection sites.Should be performed 1 to 2 months after the last dose of the HepB vaccine series using a quantitative method that allows detection of the protective concentration of anti-HBs .§A responder is defined as a person with anti-HBs greater than or equal to 10 mIU/mL after 3 or more doses of HepB vaccine.¶A nonresponder is defined as a person with anti-HBs less than 10 mIU/mL after 2 complete series of HepB vaccine.**HCP who have anti-HBs less than 10 mIU/mL, or who are unvaccinated or incompletely vaccinated, and sustain an exposure to a source patient who is HBsAg-positive or has unknown HBsAg status, should undergo baseline testing for HBV infection as soon as possible after exposure and follow-up testing approximately 6 months later. Initial baseline tests consist of total anti-HBc testing at approximately 6 months consists of HBsAg and total anti-HBc.